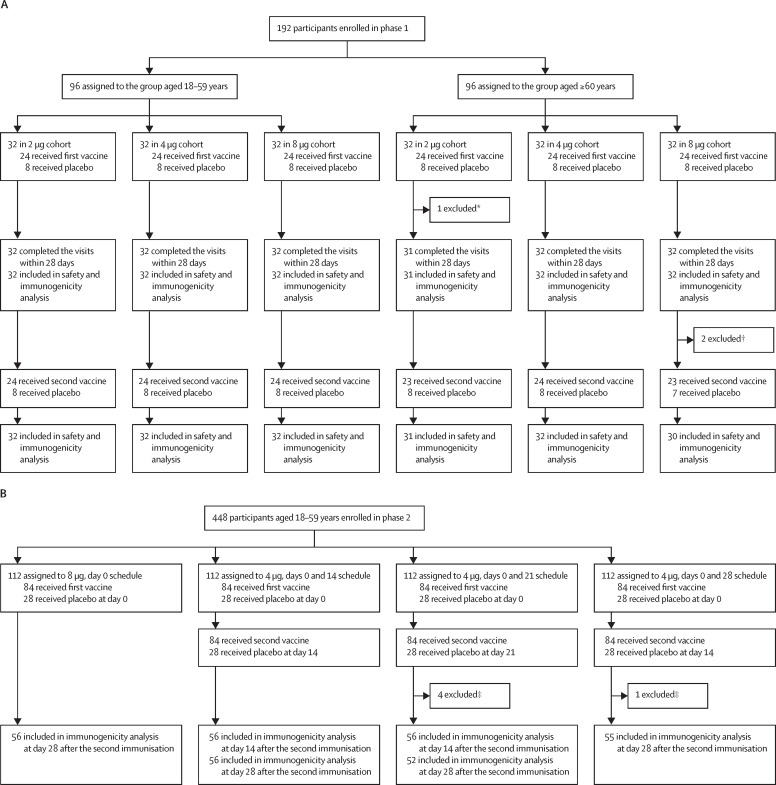
Figure 1.
Trial profile for phase 1 (A) and phase 2 (B)
*Participant received the first vaccination, finished all safety visits, but did not have blood sample taken on personal request. †Two participants quit the trial at day 28 before the second vaccination on personal request. ‡Four participants in the days 0 and 21 schedule and one participant in the days 0 and 28 schedule quit or did not finish taking blood sample at day 28 on request.