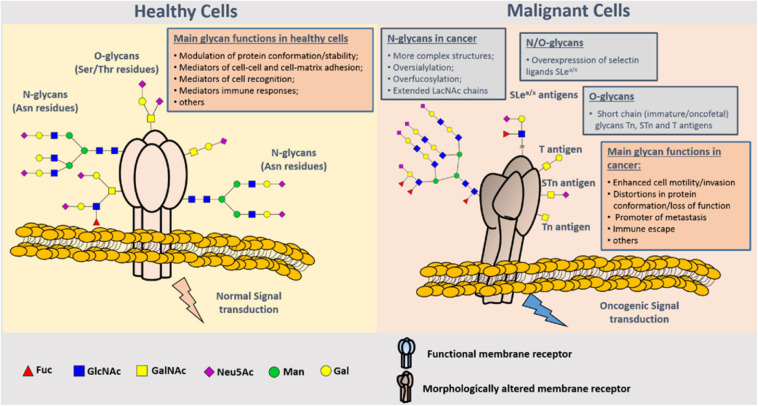
FIGURE 4.
Representation of protein N- and O-glycosylation in healthy tissues and malignant cells. Protein glycosylation plays a key role in the definition of protein folding and physiological functioning. Glycans contribute to cell–cell and cell–extracellular matrix adhesion, immune cell recognition, among other key biologic processes. Glycosylation is a highly dynamic PTM resulting from the concerted and highly regulated action of several glycosyltransferases in secretory organelles that rapidly changes in response to physiological stimuli. Several N- (Asn residues) and O- (Ser/Thr residues) glycans may coexist in the same protein backbone, depending on available glycosites and conformational constrains. In comparison to healthy cells, malignant cells tend to present more complex and branched oversialylated and/or fucosylated N-glycans. N-glycans may also be more extended by LacNAc chains. Conversely, more malignant clones present less complex and immature O-glycans, namely the Tn, sTn, and T antigens that may be also found in oncofetal tissues. Some cancer cells may also express selectin ligands sLea/x as terminal structures of both N- and O-glycans. These structural alterations at the cell-surface favor more motile and plastic cell phenotypes, invasion, lymphatic and hematogenous dissemination, and immune evasion. By impairing normal functions of cell-surface receptors, cancer-associated glycans also interfere with normal intracellular signaling transduction pathways toward the activation of oncogenic features (37).