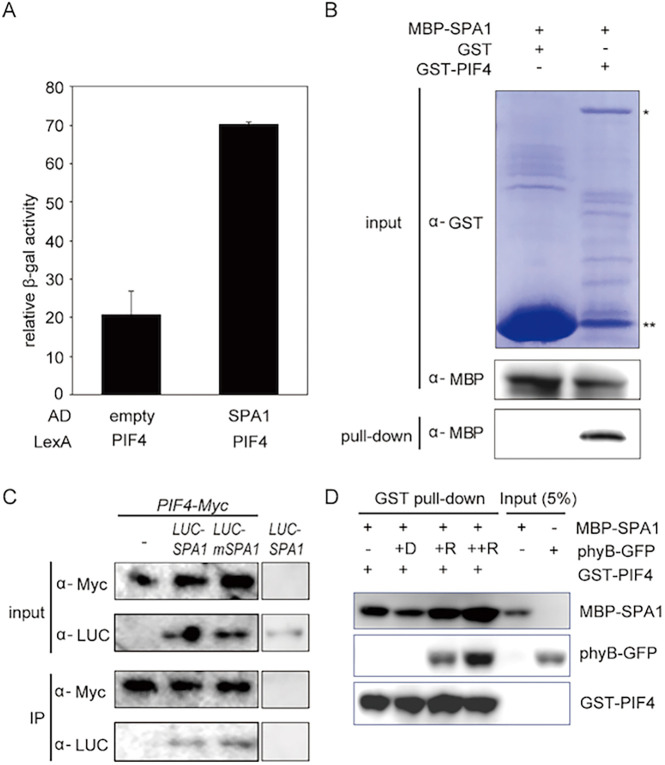
Fig. 4.
SPA1 interacts with PIF4 and forms an SPA1-PIF4-phyB ternary complex. (A) SPA1 interacts with PIF4 in yeast two-hybrid assays. LexA-PIF4 was co-transformed with empty AD or AD-SPA1. The error bars represent the s.d. Three biological replicates were used in this experiment. (B) SPA1 interacts with PIF4 in in vitro pull-down assays. GST-PIF4 and GST only (as a control) was used to pull down MBP-SPA1. Proteins were detected using anti-MBP and anti-GST antibodies. *GST-PIF4 band; **GST-only protein. (C) SPA1 interacts with PIF4 in vivo. Double transgenic plants expressing 35S:PIF4-myc with 35S:LUC-SPA1 or LUC-mSPA1 were used for this assay. 35S:PIF4-myc and 35S:LUC-SPA1 only were used as controls. PIF4-myc was used as a bait to co-immunoprecipitate LUC-SPA1 or LUC-mSPA1 and then detected using anti-LUC and anti-myc antibodies. (D) SPA1 forms a ternary complex with PIF4 and phyB in vitro. The in vitro pull-down assay shows that phyB enhances PIF4-SPA1 interaction in a light- and concentration-dependent manner. GST-PIF4 was used as a bait to pull-down MBP-SPA1 without and with an increasing amount of phyB. The samples were exposed to red light or kept in the dark. Precipitated proteins were detected using anti-MBP, anti-GFP or anti-GST HRP-conjugated antibodies.