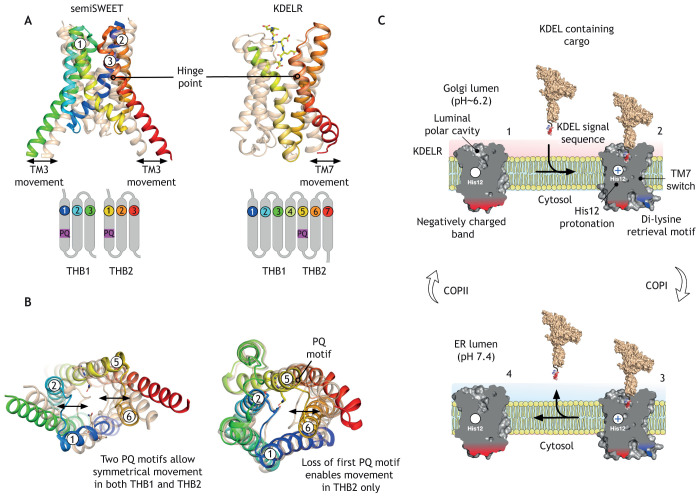
Fig. 5.
The KDELR and SWEET transporters share similar structural and mechanistic framework. (A) Crystal structures of the semiSWEET (PDBe entries:4×5n, 4×5m) and KDELR (PDBe entries: 6i6b, 6i6h) are shown overlaid. In the symmetrical semiSWEET proteins, transport results in movement in both three-helix bundles (THBs). This contrasts with the KDELR, where activation results in movement in only the second THB, which contains the PQ-motif. (B) View of structures shown in A rotated 90°. (C). The retrieval cycle of the KDELR can be viewed as a one-half cycle of a transporter. In the Golgi, the receptor is protonated at His12, creating a high-affinity binding site for the KDEL-retrieval sequence (1). Binding to KDEL-tagged cargo triggers the formation of a stable receptor–cargo complex and incorporation into COPI vesicles (2). Following trafficking to the ER (3), the receptor–cargo complex disassociates following release of the proton in the neutral pH environment (4), effectively reversing the previous step. Finally, the deprotonated receptor returns to the Golgi via the COPII system to complete the cycle (1). Panel C was reproduced from Bräuer et al., 2019.