Figure 7.
GAPDH as a DMDF Accelerates Population Expansion of Satellite Cells In Vivo
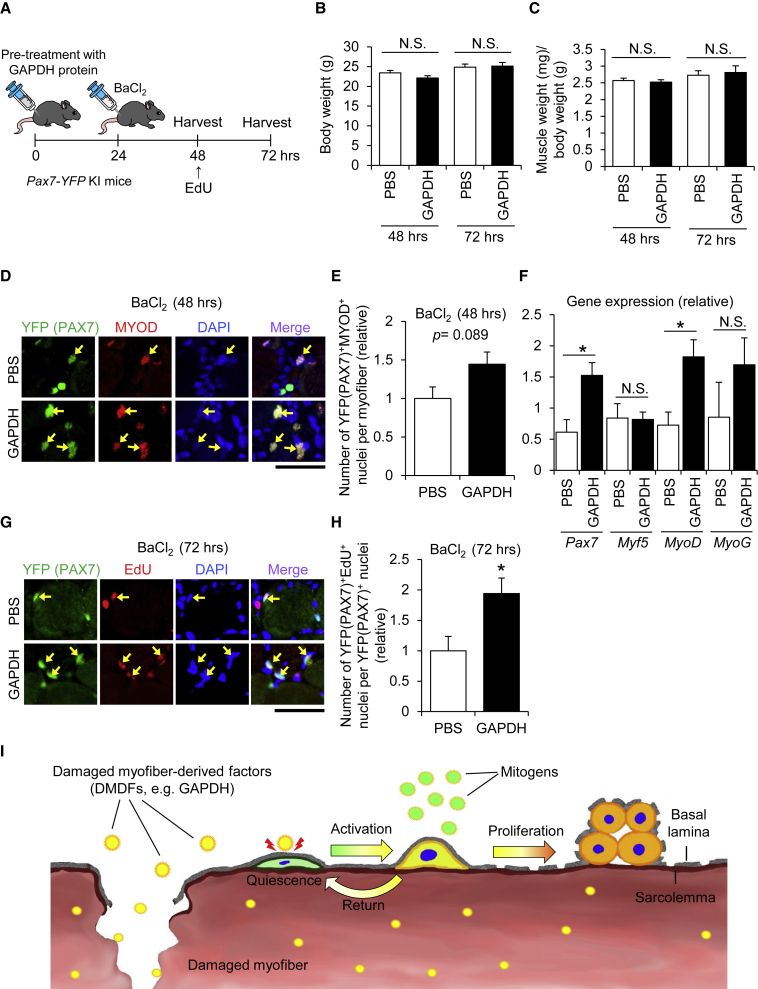
(A) A schematic illustrating the experimental procedure. To investigate the effect of DMDFs on population expansion of satellite cells in vivo, TA muscles were pre-treated with GAPDH recombinant protein, followed by BaCl2 injection to induce muscle regeneration. EdU was intraperitoneally injected into mice 24 h before sacrifice. PBS was used as a control.
(B and C) (B) Body weight and (C) TA muscle weight of mice at 48 and 72 h after treatment with GAPDH recombinant protein.
(D and E) (D) Immunohistochemistry of TA cross sections for yellow fluorescent protein (YFP) (PAX7) and MYOD (quantified in E). Arrows indicate YFP (PAX7)+/MYOD+ nuclei. Scale bars, 50 μm. Values are means ± SE (n = 3–5 mice per condition).
(F) qPCR analysis for the expression of Pax7, Myf5, MyoD, and Myogenin (MyoG) mRNAs in TA muscle tissues. Values are means ± SE (n = 3–5 mice per condition). Asterisk (∗) indicates differences compared with PBS control (p < 0.05).
(G and H) (G) Immunohistochemistry of TA cross sections for YFP (PAX7) and EdU (quantified in H). Arrows indicate YFP (PAX7+)/EdU+ nuclei. Scale bars, 50 μm. Values are means ± SE (n = 5 mice per condition). Asterisk (∗) indicates differences compared with PBS control (p < 0.05).
(I) Postulated roles of DMDFs in muscle damage and regeneration. Skeletal muscles abundantly contain metabolic enzymes such as GAPDH. Theoretically, these metabolic enzymes leak from damaged myofibers and immediately stimulate the entry of satellite cells into the G1 phase. The satellite cells are then able to proliferate extensively when subsequently stimulated by mitogens in the regenerating niche. Meanwhile, activated satellite cells also return to a quiescent-like state in the absence of mitogens, which is a multi-step protective mechanism that avoids unnecessary cell division of satellite cells.