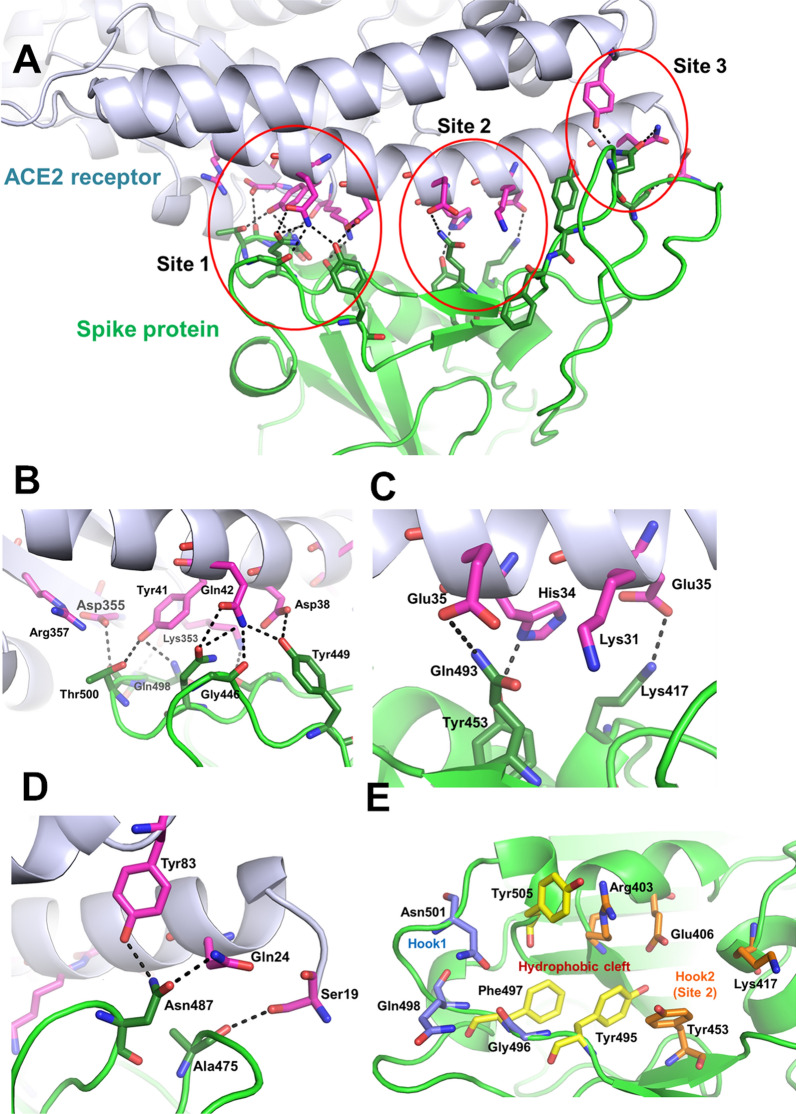
Figure 2.
The interface between the viral spike protein and ACE2. (A) Cartoon representation of the Spike-ACE2 protein interactions (in green and light blue ribbons, respectively). The interaction surface is divided into three regions – ‘Site 1’, ‘Site 2’, and ‘Site 3’. The interaction residues are shown as sticks (pink – ACE2 receptor; green – Spike protein). (B), (C) and (D) The interactions between Spike protein and ACE2 receptor are shown for the regions ‘Site 1’, ‘Site 2’, and ‘Site 3’. (E) The residues in and around ‘Site 1’ and ‘Site 2’ form a good binding site for ligands interactions. The topology for the binding site can be divided as the hydrophobic cleft, ‘hook 1’, and ‘hook 2’ regions. The hydrophobic cleft is a hotspot for the binding of compounds with strong aromatic cores. The hook1 and hook2 regions, which comprise of hydrophilic interactions, provide good support to increase interactions.