Fig. 4.
Dynein-2, the retrograde motor.
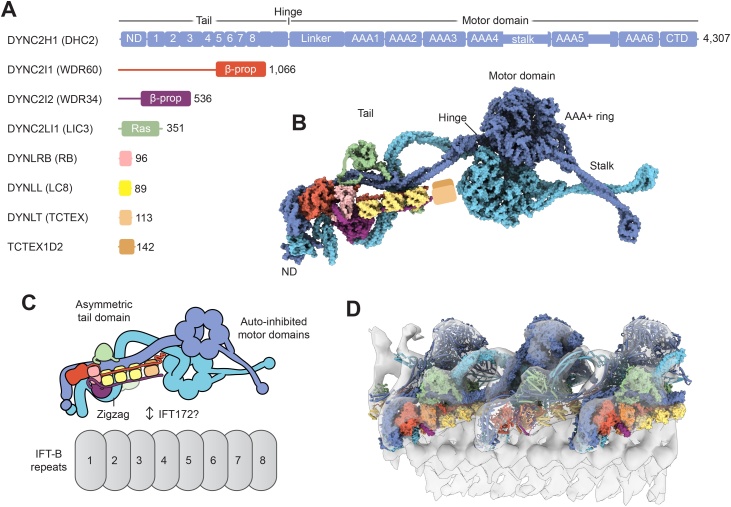
(A) Domain organization of the human dynein-2 subunits. The heavy chain (DYNC2H1) is divided into a tail and motor domain. The tail consists of a N-terminal domain (ND) and a series of α-helical bundles [49]. The motor region contains a lever-like linker domain, six AAA+ modules (AAA1−6) and a C-terminal domain (CTD). AAA1 is the ATPase site that drives dynein-2 movement, whereas AAA2 and AAA3−4 are ATP and ADP binding sites respectively [51]. The microtubule-binding domain lies at the tip of a coiled-coil stalk. The canonical isoform of human DYNC2H1 is 4307 amino acids; a non-canonical isoform featuring a 7 amino acid insertion in AAA5 also exists. Trypanosomatids feature two distinct dynein-2 heavy chains that form a heterodimer [129]. (B) Cryo-EM structure of the human dynein-2 complex [49]. Subunits are shown in surface representation, except for the flexibly attached subunits DYNLT and TCTEX1D2. (C) Diagram showing how the asymmetric tail domain and auto-inhibited motor domains of dynein-2 spread out over ∼8 IFT-B repeats. (D) Docking of the dynein-2 cryo-EM structure (PDB-6SC2) [49] into the sub-tomogram average of IFT-B and dynein-2 (EMD-4303; transparent isosurface) [28]. Individual dynein-2 complexes are shown in alternating surface and cylinder representation for distinction. Adapted from [49].