Abstract
To our knowledge, this is the first reported case of spontaneous regression of squamous cell carcinoma within a lymph node. We speculate that prior dental infection, fever, and biopsy incited an antitumor immune reaction.
Keywords: dental infection, fine needle aspiration, spontaneous regression, squamous cell carcinoma
To our knowledge, this is the first reported case of spontaneous regression of squamous cell carcinoma within a lymph node. We speculate that prior dental infection, fever, and biopsy incited an antitumor immune reaction.
1. INTRODUCTION
Spontaneous regression, the partial or complete disappearance of a malignant tumor proven by microscopic examination in the absence of treatment, is rare especially in the head and neck. We describe a case report of a 78‐year‐old man with nodal metastasis of squamous cell carcinoma (SCCa) which resolved without treatment.
The head and neck region is a frequent site for primary cancers, accounting for ~500 000 new cases annually worldwide. 1 In the United States, it accounts for 3%‐4% of malignancies, with ~50 000 Americans developing head and neck cancer annually and more than 10 000 deaths. 2 Malignancy rate is influenced by geographic variations and key risk factors. Risk factors include tobacco and alcohol use, human papillomavirus, and Epstein‐Barr virus infection. 3 For cutaneous SCCa, specific risk factors include skin type, sun exposure, age, and immunosuppression. Immunosuppression plays a major role with solid organ transplant recipients demonstrating 65‐ to 100‐fold increased cutaneous SCCa risk compared with the general population. 4 Approximately 2% of cutaneous SCCas metastasize to regional lymph nodes or more distant sites. 5
Spontaneous regression is the partial or complete disappearance of a malignant tumor proven by microscopic examination in the absence of treatment. Spontaneous regression is rare—the most common neoplasms to regress are melanoma, renal cell carcinoma, and neuroblastoma. 6 Spontaneous regression of a primary SCCa in the oral cavity has been reported; however, there have been no reports to our knowledge that identified regression of locoregionally advanced cutaneous SCCa. 7 , 8 We present a patient with history of prior cutaneous SCCa resection with regional lymph node spread which regressed without any medical treatment over a period of 5 months in the setting of a dental infection and fine needle aspiration (FNA).
2. CASE REPORT
A 78‐year‐old man with a surgical history of Mohs‐treated right auricular SCCa and right cheek basal cell carcinoma 1 year prior presented with a right neck mass. His past medical history was pertinent for atrial fibrillation, celiac disease, gout, hypertension, hyperlipidemia, and prostate cancer. He presented initially to an urgent care with a 3‐month history of slowly progressive, enlarging right neck mass with new symptoms of pain and tenderness over a month in the setting of right maxillary teeth extraction for a dental abscess.
He did not report associated symptoms of dysphagia, dyspnea, or recent upper respiratory infection. Laboratories were notable for an elevated white blood count of 13.3 × 109/L. On physical examination, he had tender, enlarged right anterior cervical lymph nodes. Given his recent dental extraction, his neck swelling was thought to be reactive lymphadenopathy, and he was discharged home with a 10‐day course of amoxicillin. Lymphadenopathy did not resolve after the antibiotic course; therefore, given his history of cutaneous malignancy, cervical metastasis was suspected.
Positron emission tomography/computed tomography (PET/CT) scan demonstrated an enlarged 1.6 × 1.2 cm right level 2 node with moderate fluorodeoxyglucose (FDG) uptake (Figure 1). Two weeks after his imaging, he underwent a FNA biopsy with pathology demonstrating SCCa (Figure 2). Direct laryngoscopy did not identify any aerodigestive tract lesions, and base of tongue biopsies was benign. The patient’s case was presented at our multidisciplinary tumor board which recommended a right selective neck dissection.
Figure 1.
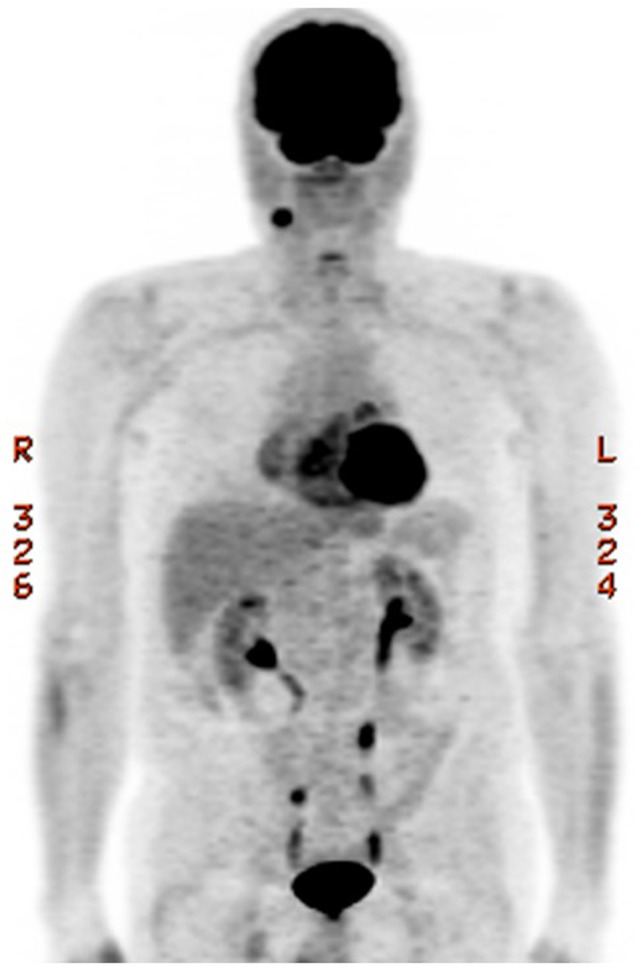
Positron emission tomography (PET) scan demonstrated an enlarged 1.6 × 1.2 cm right level 2 node with moderate fluorodeoxyglucose (FDG) uptake
Figure 2.
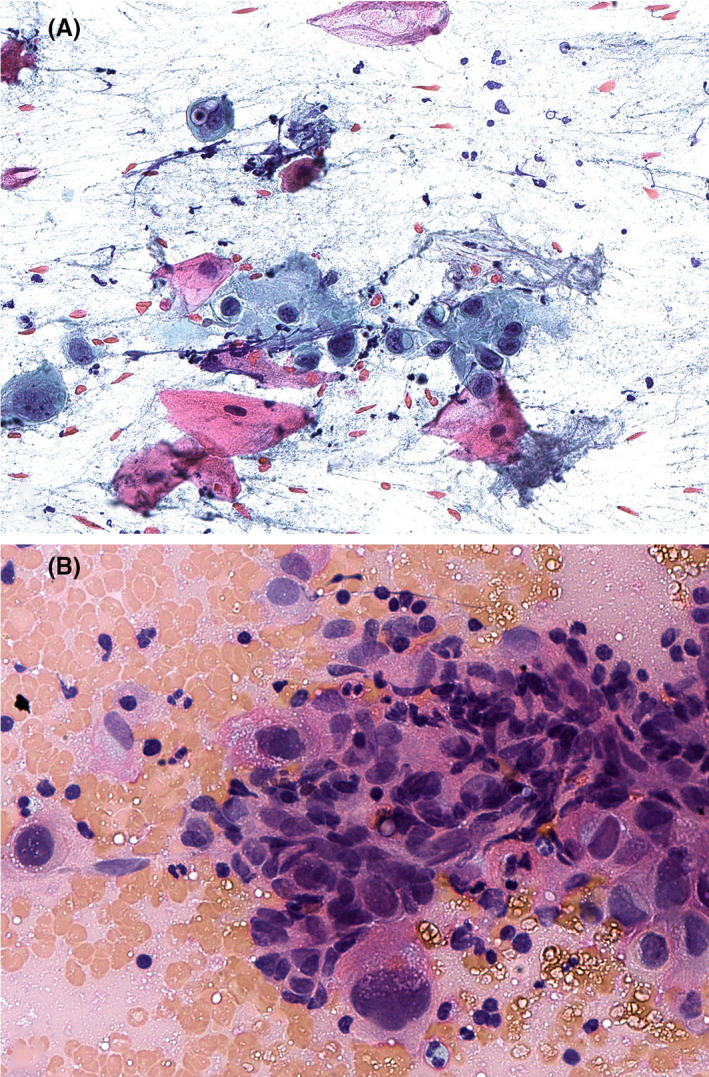
A, Fine needle aspiration (FNA) biopsy of the right cervical mass revealed a hypercellular sample composed of atypical squamous cells, singly and in groups, with variable keratinization (pink to orange cytoplasm) in a background of scattered inflammatory cells (Papanicolaou stain). B, The squamous cells demonstrated marked atypia including enlarged nuclei with irregular nuclear contours, increased nuclear‐to‐cytoplasmic ratios, and irregularly distributed chromatin with hyperchromasia, indicative of squamous cell carcinoma (Giemsa stain)
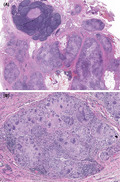
Twelve weeks after the initial PET/CT scan and 10 weeks after the FNA biopsy, the patient noted that his right neck mass was barely palpable and an updated CT scan confirmed presence of level 2 mass with an irregular border, but with significant interval size decrease from 12 mm to 6 mm in the short axis compared to the prior PET/CT scan (Figure 3). Decision was made to proceed with a selective neck dissection of level 2A, 2B, and 3. Histopathology of 1 of 9 level 2 nodes demonstrated an architecturally abnormal lymph node with fibrosis and a multinucleated giant cell reaction to keratin debris without any viable tumor cells identified (Figure 4). Immunohistochemistry showed that the immune cells surrounding these regions were predominantly composed of histiocytes and T cells without many B cells or NK cells (Figure 5). All other lymph nodes were benign and histologically unremarkable. Given the lack of malignancy in the cervical node, further treatment was not advised. Recommendation was made for a repeat staging PET/CT scan 3 months postoperatively; however, the patient did not want to pursue further imaging. Eleven months postoperatively, the patient does not report clinical return of symptoms of neck swelling or mass.
Figure 3.
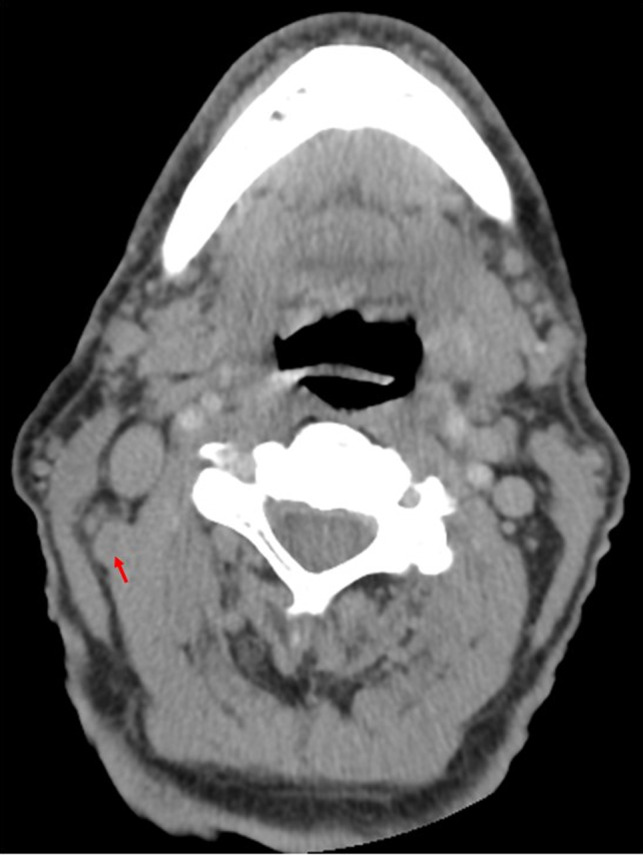
Computed tomography (CT) scan demonstrated significant decrease in size of right level 2 node from 12 mm to 6 mm in the short axis marked by the red arrow
Figure 4.
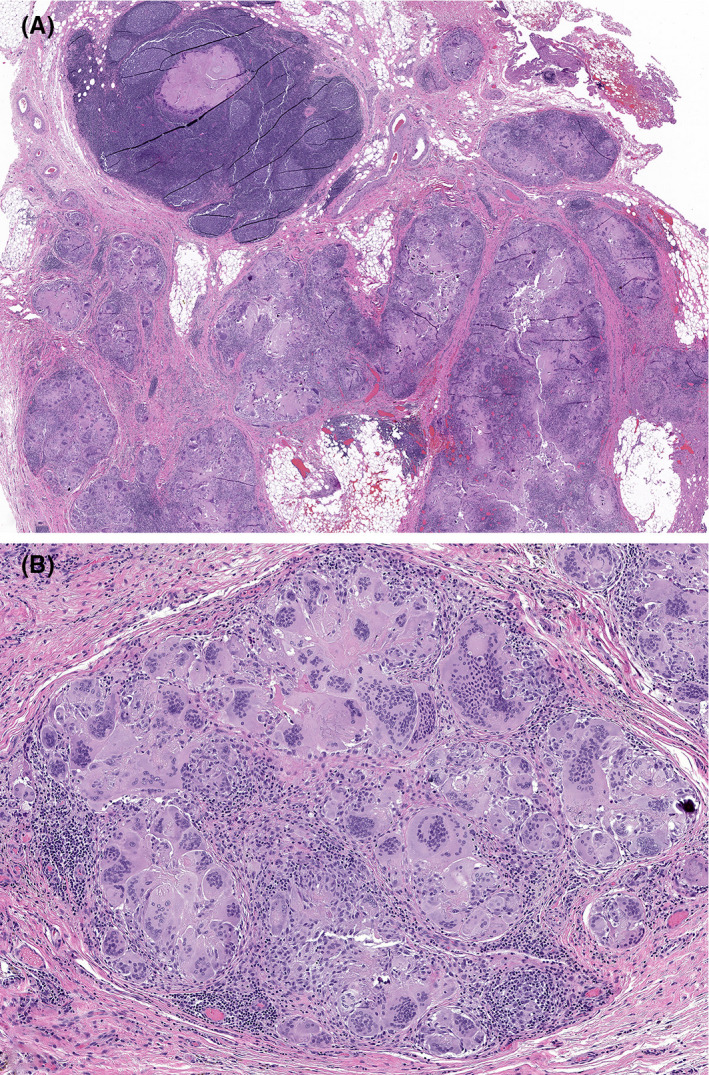
A, A low‐power view of a histologic section from the resected level 2 lymph node showed abnormal lymph node architecture in the form of peripheral multinodularity and intranodal fibrosis (hematoxylin and eosin stain). B, On higher power, the nodules were composed of abundant mono‐ and multinucleated histiocytes forming a multinucleated giant cell reaction to keratin debris. No viable tumor cells or necrotic “ghosts” of tumor cells were identified within the specimen (hematoxylin and eosin stain)
Figure 5.
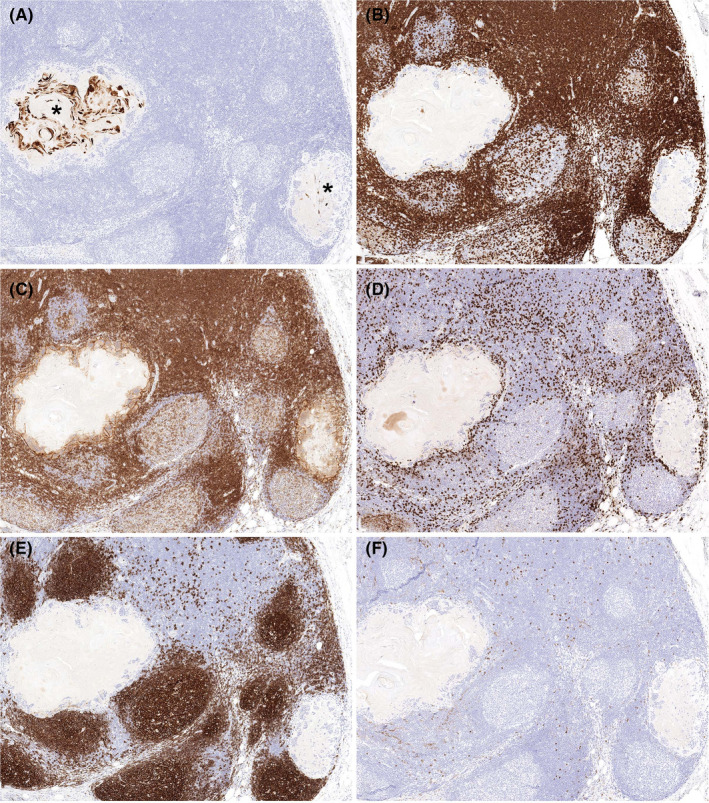
A, Immunohistochemistry for cytokeratin highlighted variable amounts of keratin debris within different nodules of the abnormal lymph node (asterisks, immunohistochemical stain). While there was a paucity of inflammatory cells within these nodules, their peripheral rims were characterized by CD3+ T cells (B) that were a mixture of CD4+ (C) and CD8+ (D) subtypes (immunohistochemical stains). The prominent histiocytic reaction was also highlighted by CD4 immunohistochemistry (C). CD20+ B cells were predominantly restricted to the underlying native germinal centers (E), and only scattered CD56+ NK cells were present, without an apparent increase adjacent to the keratin granulomas (F) (immunohistochemical stains)
3. DISCUSSION
Spontaneous regression has been defined in the literature as partial or complete disappearance of a tumor in the absence of treatment. 9 The incidence of spontaneous regression for neoplasms is estimated to be one in every 60 000‐100 000 cases of cancer. 6 , 10 Little is reported regarding spontaneous regression of head and neck cancer. The first reported case was a patient with SCCa of the left tonsil and base of tongue in 1910, and there are recent reports of floor of mouth and tongue SCCa resolutions. 7 , 8 , 11 To our knowledge, spontaneous cervical lymph node metastasis resolution has only been reported once in the head and neck literature; in that report, the size of the metastatic oropharyngeal SCCa deposit decreased radiographically over the course of weeks after the FNA biopsy, and histologic examination of the subsequently resected lymph node showed residual SCCa with evidence of apoptosis. 9 In contrast, our current case showed complete histologic tumor regression, with morphologic features similar to those seen in tumor regression after neoadjuvant chemoradiotherapy. 12 , 13 , 14
We also considered the possibility of a false‐positive FNA biopsy of a benign intranodal process. However, the degree of cytologic atypia present in the preoperative FNA specimen exceeded that seen in the reactive atypia of inflamed, benign squamous epithelial‐lined cysts. Similarly, the subsequent lymph node excision specimen showed multinodular architecture with fibrosis and keratin granulomas, findings characteristic of regressed carcinoma, rather than preserved nodal architecture with a limited epithelial proliferation (as in a benign intranodal epithelial inclusion) or a cystic structure with lymphoid stroma lacking lymph node architecture (as in benign developmental cysts such as branchial cleft cysts).
Mechanisms of tumor regression are largely unknown. Explanations for this phenomenon include immune modulation, removal of carcinogenic substances, or infection. 6 In this patient’s case, our hypothesis is that the dental infection and/or subsequent FNA triggered an immune response. Within a lymph node metastasis, the presence of a native lymphoid stroma complicates one’s ability to assess for the subsets of inflammatory cells that may have led to tumor regression. 15 In our case, predominantly histiocytes and both CD4+ and CD8+ T cells were present adjacent to the keratin granulomas. Infections elicit a strong immunological response and fever, and the presence of a fever has been recorded in up to 80% of patients with spontaneous tumor regression cases—many of which presented with severe systemic illnesses such as malaria, measles, or tuberculosis. 16 Similarly, biopsies have been shown to induce local, and to some degree, systemic inflammation resulting in tumor resolution. 17 Further studies are required, but prior reports suggest that pyrogenic reaction is correlated with an induced immune response which has potential in eliminating tumor burden and leading to tumor resolution. 16
4. CONCLUSION
To our knowledge, this is the first reported case of locoregionally advanced cutaneous SCCa that underwent spontaneous regression. Clinically, this phenomenon occurred in the context of a local infection, fever, and prior biopsy. This case, together with a review of previously published literature, highlights consideration of induction of a pyrogenic reaction as a therapeutic tool in the adjuvant treatment of locoregionally advanced head and neck cancers.
CONFLICT OF INTEREST
None of the authors have any financial or personal conflicts of interest.
AUTHOR CONTRIBUTION
PD, MD: contributed to revision of manuscript and critical review of literature. MJK and MP, MD: involved in initial drafting of manuscript and initial conception and design of report. DAK, MD: contributed to revision of manuscript and to pathological analysis, interpretation, literature review, and acquisition of pathology slides. JAP, MD: contributed to conception and design of case report and final approval of manuscript and accountable to accuracy and integrity of work.
ACKNOWLEDGMENTS
None.
Divakar P, Khan MJ, Polacco M, Kerr DA, Paydarfar JA. Spontaneous regression of squamous cell carcinoma in the setting of dental infection and needle biopsy. Clin Case Rep. 2020;8:1919–1923. 10.1002/ccr3.3024
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7‐34. [DOI] [PubMed] [Google Scholar]
- 3. Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res. 1998;18(6 B):4779‐4786. [PubMed] [Google Scholar]
- 4. Chockalingam R, Downing C, Tyring S. Cutaneous squamous cell carcinomas in organ transplant recipients. J Clin Med. 2015;4(6):1229‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brougham NDLS, Dennett ER, Cameron R, Tan ST. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012;106(7):811‐815. [DOI] [PubMed] [Google Scholar]
- 6. Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17(3):201‐209. [DOI] [PubMed] [Google Scholar]
- 7. de Andrade Sousa A, Lopes Rena R, Souza Silva G, et al. Spontaneous remission of a squamous cell carcinoma of the floor of the mouth. J Craniomaxillofac Surg 2014;42:1536‐1539. [DOI] [PubMed] [Google Scholar]
- 8. Oya R, Ikemura K. Spontaneous regression of recurrent squamous cell carcinoma of the tongue. Int J Clin Oncol. 2004;9(4):339‐342. [DOI] [PubMed] [Google Scholar]
- 9. Kurita M, Hirano K, Ebihara S, et al. Spontaneous regression of cervical lymph node metastasis in a patient with mesopharyngeal squamous cell carcinoma of the tongue: possible association between apoptosis and tumor regression. Int J Clin Oncol. 2007;12(6):448‐454. [DOI] [PubMed] [Google Scholar]
- 10. Chang WY. Complete spontaneous regression of cancer: four case reports, review of literature, and discussion of possible mechanisms involved. Hawaii Med J. 2000;59(10):379‐387. [PubMed] [Google Scholar]
- 11. Godfrey F. Spontaneous cure of cancer. Br Med J. 1910;2(2):2027. [Google Scholar]
- 12. Tanner NS, Carter RL, Dalley VM, Clifford P, Shaw HJ. The irradiated radical neck dissection in squamous carcinoma: a clinico‐pathological study. Clin Otolaryngol Allied Sci. 1980;5(4):259‐271. [DOI] [PubMed] [Google Scholar]
- 13. Bollschweiler E, Hölscher AH, Metzger R, et al. Prognostic significance of a new grading system of lymph node morphology after neoadjuvant radiochemotherapy for esophageal cancer. Ann Thorac Surg. 2011;92(6):2020‐2027. [DOI] [PubMed] [Google Scholar]
- 14. Westra WH, Forastiere AA, Eisele DW, Lee D. Squamous cell granulomas of the neck: histologic regression of metastatic squamous cell carcinoma following chemotherapy and/or radiotherapy. Head Neck. 1998;20(6):515‐521. [DOI] [PubMed] [Google Scholar]
- 15. Hendry S, Salgado R, Gevaert T, et al. Assessing tumor‐infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno‐Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non‐small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24(6):311‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas JA, Badini M. The role of innate immunity in spontaneous regression of cancer. Indian J Cancer. 2011;48(2):246‐251. [DOI] [PubMed] [Google Scholar]
- 17. Matsui T, Mizuno T, Kuroda H, et al. Spontaneous regression of lung squamous cell carcinoma with synchronous mediastinal progression: a case report: spontaneous regression of lung cancer. Thorac Cancer. 2018;9:1778‐1781. [DOI] [PMC free article] [PubMed] [Google Scholar]


