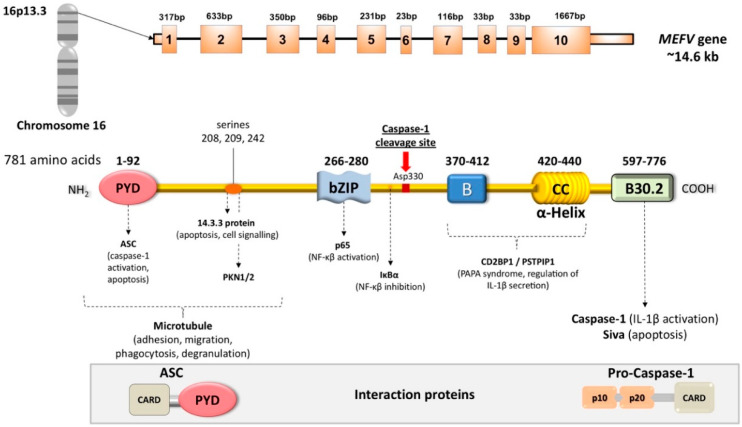
Figure 1.
Schematic structure of MEFV gene and encoded pyrin (marenostrin) protein. The MEFV gene encodes for the pyrin protein (781 amino acids). The most common mutations in Familial Mediterranean Fever (FMF) are in exon 10 encoding the B30.2 domain. The most important interaction partners appear below the pyrin structure. ASC and Pro-Caspase 1 are also drawn. Pyrin structure includes five different domains, each one responsible for protein-protein interaction, and each domain has a role in the regulation of innate response. From left to right, PYRIN (PYD) domain (residues 1–92) interacts with ASC (apoptosis-associated speck-like protein containing a CARD—caspase-recruitment domain). bZIP transcription factor basic domain (residues 266–280) interacts with the p65 subunit (transcription factor p65) of NF-κB, and IκB-α. The B-box zinc finger domain (residues 375–412) and α-helical (CC, coiled-coil) domain (residues 420–440) likely influence the oligomerization of pyrin, and interact with the PAPA protein (also named PSTPIP1, proline serine threonine phosphatase-interacting protein, also known as CD2BP1 involved in the organization of the cytoskeleton) and the regulation of IL-1β secretion. The B30.2 domain (PRYSPRY) (residues 597–776) is the most important, and interacts with caspase-1 and the proapoptotic protein Siva. Further pyrin interactions include binding to microtubules (starting from the N-terminal to bZIP), interaction with 14.3.3 (14-3-3 protein), and with the PKN1/2 (serine-threonine kinases PKN1 and PKN2) at the three serine residues 208, 209, 242 between PYD and bZIP. The position of Asp330 between bZIP and the B-box indicates the caspase-1 cleavage site. Mutations in the B30.2 domain tend to be transmitted in an autosomal-recessive fashion. Mutations in exons 2, 3 and 5 generally exhibit autosomal-dominant pattern of inheritance [24,34,35,36,41,43,48,49,50,51,52,53,54,55,56,57,58].