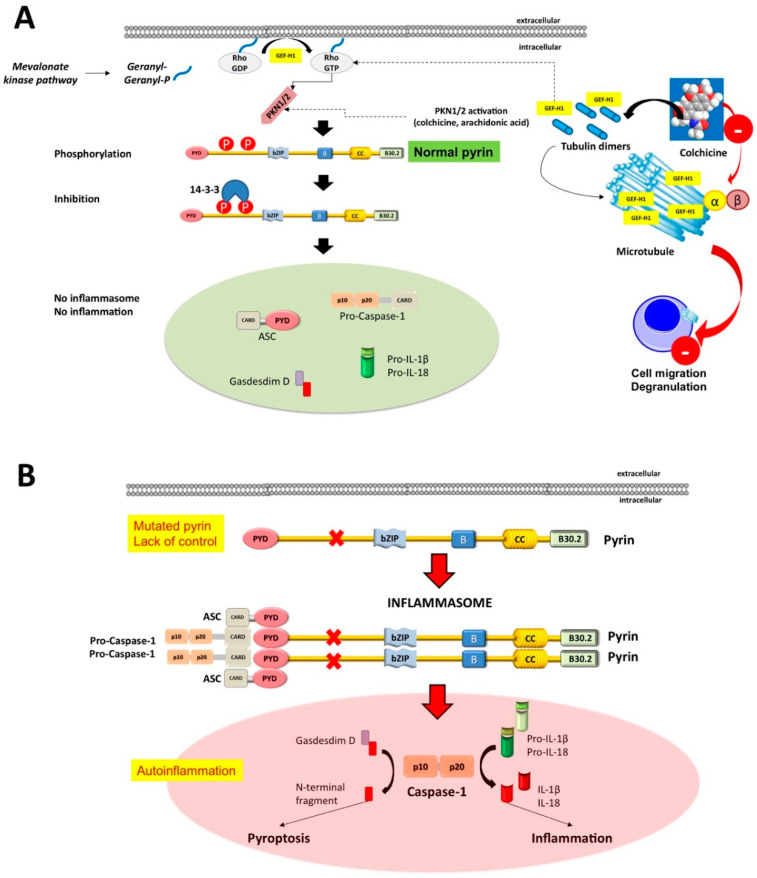
Figure 2.
Mechanisms underlying the assembly of the pyrin inflammasome. (A) With a normally functioning pyrin (i.e., when pyrin is non-mutated), the mevalonate kinase pathway provides geranyl-geranyl phosphate and, together with release of GEF-H1 (increased by colchicine acting as the inhibitor of the microtubule polymerization), activates RhoA. PKN1 and PKN2 are effector kinases of RhoA mediating the phosphorylation of pyrin and binding to the inhibitory proteins 14-3-3. Inhibition of pyrin can increase with agents activating PKN1/2 or following the release of GEF-H1 (i.e., colchicine). (B) If the pyrin phosphorylation decreases, (i.e., in FMF patients lacking the control by pyrin/marenostrin caused by pathogenic MEFV variants), with low GEF-H1 or defective function of the MVK-pathway, the activation of PKN1/2 also decreases. This step results in pyrin inflammasome activation and release of mature IL-1β and IL-18. The plasma membrane pore-forming N-terminal fragment of gasdermin D facilitates IL-1β and IL-18 release. Appropriate stimuli can also lead to the assembly of the inflammasome. The first step is the PYD-PYD homotypic interaction of ASC resulting in oligomerization into ASC specks. Pro-caspase-1 is recruited because of CARD-CARD interaction with ASC. This step anticipates the auto-cleavage of pro-caspase-1 into active caspase-1 tetramers (p10/p20) governing the transformation of pro-IL-1/18 into mature IL-1/18. The pyroptosis mediated by Gasdermin D also contributes to cytoplasmic enrichment with IL-1/18, and further reinforces the inflammatory pathway. Colchicine inhibits the polymerization of intracellular β-tubulin by forming colchicine-tubulin complexes via contact of A and C rings with the C domain of the tubulin β-subunit. These supramolecular interactions block the dockage of tubulin into the (+) ends of microtubules (cytoskeleton), thus preventing inflammasome activation in neutrophils and monocytes. The colchicine-dependent inhibition of tubulin also efficiently affects the migration and degranulation of white blood cells [43,50].