Abstract
Simple Summary
Ludwigia species have several pharmacological applications, but their insecticidal proprieties have not been tested. This research thus aimed to study the effects of aqueous extracts on the biological characteristics of Plutella xylostella. We noted that the L. tomentosa, L. longifolia and L. sericea extracts were active. These species showed the best results regarding their ability to control P. xylostella populations, due to the presence of substances that inhibit food consumption and interfere in the morphological and physiological transformations of the offspring and adult oviposition.
Abstract
We tested the bioactivity of aqueous extracts of Ludwigia spp. (Myrtales: Onagraceae) on the biological cycle of Plutella xylostella. We assessed the duration of and viability during the larval, pupal and adult phases, as well as the influence of the extracts on the fecundity and hatching of P. xylostella eggs. Subsequently, we phytochemically screened the extracts. The extracts of L. tomentosa and L. longifolia reduced the pupal weight instead of prolonging the larval stage of P. xylostella. The L. tomentosa effect caused higher larval mortality and reduced the fecundity and hatching of P. xylostella eggs, and L. sericea reduced the egg survival. The phenolic compounds—flavonoids, condensed tannins and alkaloids—were more abundant in L. nervosa, L. tomentosa, L. sericea and L. longifolia. The L. tomentosa, L. longifolia and L. sericea extracts were bioactive, and these species showed the best results regarding their ability to control P. xylostella populations, because these plants produce substances able to inhibit food consumption and interfere with the morphological and physiological transformations of the offspring and the oviposition of adults.
Keywords: Ludwigia tomentosa, Ludwigia longifolia, Ludwigia sericea, Ludwigia nervosa, diamondback moth
1. Introduction
The Plutella xylostella (Linnaeus, 1758) (Lepidopera: Plutellidae) microlepidoptera, popularly known as the diamondback moth, is the most economically important pest for the Brassicaceae family. Around four to five billion US dollars are spent on its control worldwide [1]. Due to huge economic damage, chemical control is the best way to contain P. xylostella [2]. In some parts of the world, 20 applications of synthetic insecticides have been registered in a single growing season.
The existence of synthetic insecticides with various formulations have increased crop yields, but their extensive use to control P. xylostella has resulted in more rigid selection, leading to the development of resistance to more than 95 different insecticides [3].
If incorrectly used, synthetic insecticides can be harmful; they may be problematic for human health, cause ecological problems [4]; contaminate food products; damage the environment (contamination of water, air and soil resources); increase the resistance and favor the recurrence of pests; eliminate nontarget organisms [5,6] and harm natural enemies [7]. Therefore, it is necessary to adopt techniques and tactics that are less harmful but equally efficient for controlling P. xylostella.
Extracts of many plant species show proven insecticidal effects, since they contain diverse active compounds that act synergistically, with attractive or repellent characteristics [8,9,10,11,12,13]. Plants create and use their own secondary metabolites as protection against microorganisms, insects and other phytophagous arthropods [14]. Such compounds become natural candidates for discovering new products that can be used to control insects.
Compounds extracted from plants can be incorporated into integrated pest management (IPM) and act as contact insecticides, repellents and food and/or reproduction suppressors [15]. Herb-based products are often described as benign to beneficial arthropods and the ecosystem [16] and are rapidly decomposed after being exposed to the atmosphere [17], reducing the risk of residues remaining in food. Botanical insecticides can be selective [18] and generally do not cause any resistance, as do synthetic insecticides [19,20].
A number of studies have been performed to determine the insecticidal potential of botanical oils and extracts of certain species—Myrtaceae [21], Rubiaceae [22], Anonnaceae [23,24], Meliaceae, Anacardiaceae [24,25], Apocynaceae [26,27], Sapindaceae and Fabaceae [28]—and the oil of Rutaceae [29] on the development and/or oviposition of P. xylostella and, consequently, the occurrence of antibiosis and antixenosis.
Some species of the Ludwigia genus show promising sources of anticancer phytochemicals [30] and antioxidants [30,31,32], with antibacterial [31,32,33] and antifungal activity [34]. However, no studies have been reported showing the insecticidal potential of this genus. This study therefore tested the hypothesis that species from this genus have insecticidal potential against P. xylostella.
Upon analyzing the antibacterial and antifungal actions of L. decurrens Walter [34], L. abyssinica A. Rich. and L. leptocarpa (Nutt.) Hara, they were found to have saponins [31], and L. adscendens (L.) H. Hara was found to contain tannins, alkaloids, flavonoids, terpenes, triterpenoids and phenols.
Secondary metabolites such as flavonoids can be responsible for changes in the biological and morphological characteristics of insects [22]. They also act as phagodeterrent substances [35], similar to alkaloids [36] and tannins [37]. However, there is no report of the insecticidal potential of species of the genus Ludwigia to date.
Understanding their effects on the lifecycle of P. xylostella could improve the knowledge and encourage future investments in research into this plant genus. Our aim was, thus, to test the bioactivity of aqueous extracts of Ludwigia species against P. xylostella.
2. Materials and Methods
Extracts and bioassays were prepared in the Insect-Plant Interaction Laboratory of the Faculty of Biological and Environmental Sciences at the Federal University of Grande Dourados—UFGD, Dourados, MS, Brazil. The compounds were classified at the Center of Studies in Natural Resources of the State University of Mato Grosso do Sul—UEMS in Dourados, MS, Brazil.
2.1. Rearing of P. xylostella
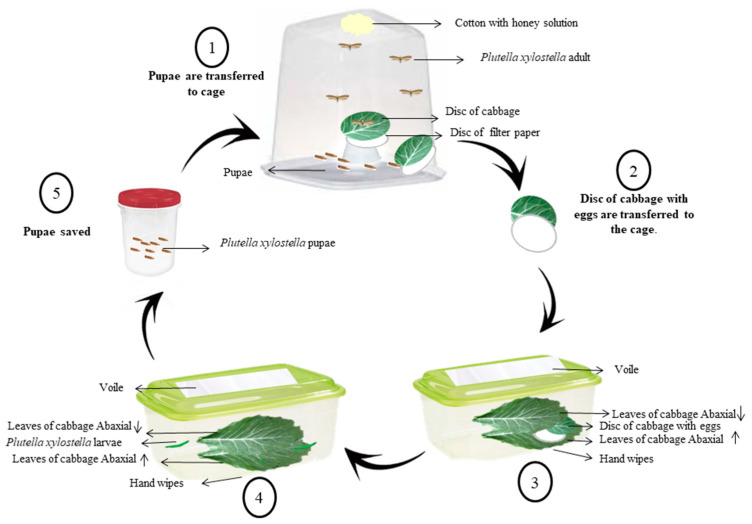
Individuals were maintained under constant temperature (25 ± 2 °C), relative humidity (55% ± 5%) and photoperiod (12 h). The pupae were placed in a transparent plastic cage (9-cm-long × 19-cm-wide × 19-cm-high) until the adults emerged, which were fed with a 10% honey solution. Cabbage leaf discs, manually cut to 8 cm in diameter, were placed on a paper filter and used as an oviposition substrate. These discs were replaced daily, and the eggs were transferred to sterile plastic pots measuring 30-cm-long × 15-cm-wide × 12-cm-high. After hatching, the larvae remained in these containers until they reached the pupal stage.
The feeding substrate of the larvae was composed of organic cabbage leaves (B. oleracea var. acephala) cleaned with 5% sodium hypochlorite solution and later washed in running water. The cabbage leaves were arranged with the adaxial face facing the plastic container and the abaxial face free. The larvae were placed on them, and then, another leaf of cabbage was placed with the abaxial side facing the larvae (Figure 1). This procedure was performed daily, always keeping the leaves higher, and was repeated until the pupae were formed [38].
Figure 1.
A schematic representation of the methodology used for rearing Plutella xylostella by Matias da Silva et al. [39].
2.2. Botanical Materials
Expanded leaves of L. tomentosa (Cambess.) H. Hara, L. longifolia (DC.) H. Hara, L. sericea (Cambess.) H. Hara and L. nervosa (Poir.) H. Hara were collected from highway surroundings in the city of Dourados, MS. The collection area was between the Atlantic Forest and the Cerrado and was beginning to regenerate (secondary succession), with a predominance of herbaceous vegetation (grasses and ferns) and shrub vegetation (22°11′54.92″ S, 54°46′52.15″ O).
The plant species were identified by a specialist in the laboratory of Applied Botany, and the specimens were deposited at the Herbarium of the Federal University of Grande Dourados—UFGD, with the following registration numbers: 6391—L. tomentosa, 6389—L. longifolia, 6388—L. sericea and 6390—L. nervosa. The collection of the botanical material was authorized by the Brazilian National Research Council (CNPq)/Council of Genetic Heritage Management (CGEN/MMA), under the number A9ECAC6.
2.3. Preparation of Aqueous Extracts
The leaves were dried inside a forced-air circulation oven for three days at a maximum temperature of 40 °C (±1 °C) and then crushed in an industrial mill to obtain a fine powder. Maceration was used to prepare the aqueous extracts. The aqueous extracts were prepared by mixing 5 g of solid plant material with 50mL of distilled water. Next, we used a paper filter to separate the solid material from the extract. The extracts were then refrigerated (10 °C) for 24 h and strained through cheesecloth. Extracts at a concentration (weight/volume) of 10% were obtained.
2.4. Bioactivity of Ludwigia spp. Aqueous Extracts against P. xylostella
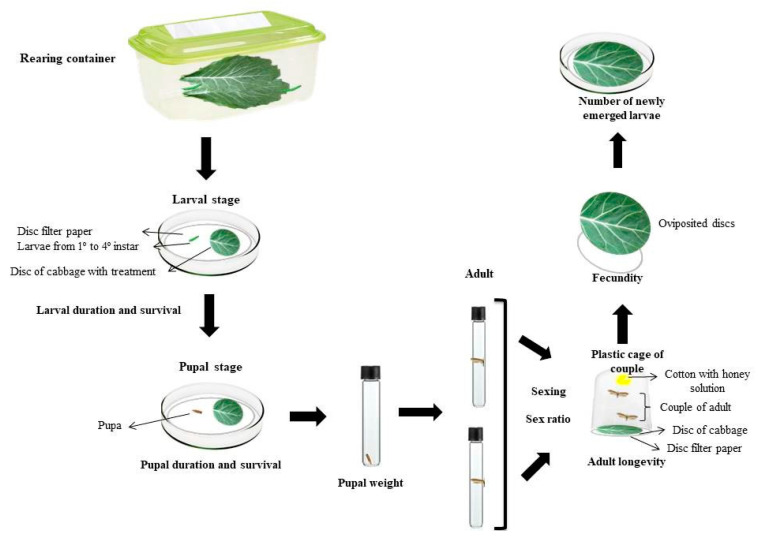
The methodology for assessing the bioactivity of the plant extracts was based on [22]. The larvae were monitored until they reached the pupal stage (larval duration). The first assessment of mortality was made at 48 h after the confinement of the larvae in the Petri dishes, counting the number of dead individuals and replacing the cabbage leaf discs with others of the same treatment. The following assessments were performed daily, and the leaf discs were changed every 24 h, until the larvae reached the pupal stage or not (larval survival).
The pupae of each treatment were separated in test tubes to assess the time spent in the pupal stage (pupal duration). The pupae were weighed 24 h after pupation (pupal biomass) (Bel Mark Analytical Balance—0.001 g). The duration of this stage was subsequently monitored until the pupae emerged, reaching adulthood or not (pupal survival).
Ten couples from each treatment were separately placed in plastic cages with cabbage leaf discs (diameter of 8 cm) acting as oviposition substrates for assessing the reproductive stage. The cabbage discs were replaced by new ones daily. The discs with the eggs were transferred to Petri dishes to count the number of eggs (fecundity) and to monitor the hatching of larvae with a Motic SMZ-168 series stereoscope.
The biological parameters assessed were the duration (days) of and survival (in %) during the larval and pupal stages, pupal weight (in mg), longevity of the females and males (days), sex ratio (sr = female/female + male), fecundity (total number of eggs laid throughout life), newly emerged larvae (numbers of eggs hatched in larvae) and survival of the eggs (percentage of eggs hatched in larvae) (Figure 2).
Figure 2.
A schematic representation of the methodology used for the evaluation of the biological parameters of Plutella xylostella.
2.5. Determination of Polyphenolic Content
The total phenolic content was determined with the Folin–Ciocalteu reagent method [40] and samples at a concentration of 1000 µg/mL. The absorbance was measured using a spectrophotometer (FEMTO 700 PLUS, FEMTO, São Paulo, São Paulo, Brazil) (ë = 760 nm). Gallic acid (Sigma-Aldrich, St. Louis, MO, USA) was used as a standard at concentrations of 5–1000 µg/mL. The results are expressed in milligrams of gallic acid per gram of dry weight of extract.
2.6. Determination of Flavonoids
The flavonoids were determined using 1000 µL of 2% AlCl3 methanol solution, which was added to 1000 µL of the extracts (1000 µg/mL). After 15 min of incubation, the absorbance of the final mixture was measured using a spectrophotometer (FEMTO 700 PLUS) (ë = 430 nm) [40]. Rutin (Sigma-Aldrich, St. Louis, MO, USA) was used as a standard at concentrations of 1–50 µg/mL. The flavonoid contents are expressed in milligrams per gram of dry weight of extract.
2.7. Determination of Condensed Tannin
For the condensed tannin test, samples (1000 µg/mL) were mixed with 5 mL of vanillin-HCl (8% aq. conc. HCl and 4% vanillin in methanol). Methanol served as the blank, and a standard curve of catechin (Sigma-Aldrich, St. Louis, MO, USA) was used. The mixture was incubated in a water bath for 20 min, and then, the absorbance was measured at 510 nm [41]. The results are expressed in catechin milligrams per gram of dry weight of extract.
2.8. Determination of Alkaloids
The total alkaloid contents in the samples were quantified according to the procedure developed by [42]. In the analysis, 40 mL of extract at a 1000-µg/mL concentration was used and acidified to pH 2–2.5 with 1-N HCl and 4 mL of Dragendorff reagent and centrifuged at 2400 rpm for 30 min. The supernatant was discarded, and the residue was treated with 1 mL of solute ethyl alcohol; 2 mL of 1% sodium sulfite was added, and the mixture was centrifuged at 2400 rpm for 30 min. The supernatant was then discarded, and the residue was treated with 2 mL of concentrated nitric acid; the resulting contents were transferred to a 50-mL volumetric flask and brought to volume with distilled water. Then, 1 mL of this solution was taken, and 5 mL of 3% (w/v) thiourea was added; the mixture of nitric acid and thiourea was used as a blank, and the sample’s absorbance read at 435 nm was measured. Berberine (Sigma-Aldrich, St. Louis, MO, USA) was employed as the standard, and linearity was obtained between 40 and 200µg/mL. The alkaloid contents are expressed in milligrams per gram of dry weight of extract.
2.9. Determination of Antioxidant Activity
The antioxidant activity of the extracts was assessed using the free radical indicator DPPH (1,1-diphenyl-2-picrilhidrazyl) (Sigma-Aldrich, St. Louis, MO, USA) [43]. The percentage of inhibition by each concentration was used to obtain the IC50 values, which were the minimum concentrations of antioxidant necessary to reduce the initial concentration of DPPH by 50%. The experiment was carried out in a room under a light shelter, with a controlled temperature (25 ± 1 °C). To calculate the minimum inhibitory concentrations (IC50), the extracts were prepared with distilled water at the following concentrations: 5, 10, 20, 200, 30, 40, 50, 60, 70, 80, 90 and 100 µg/mL. Based on the sequestering activity of the different dilutions of the sample, a graph was plotted with the % reduction of DPPH on the Y-axis and the concentration of the extracts (µg/mL) on the X-axis to determine the concentration of the sample necessary to reduce 50% of the DPPH and a correlation coefficient. The coefficients (r) obtained for the samples were 0.989 for L. tomentosa, 0.991 for L. longifolia, 0.990 for L. sericea and 0.986 for L. nervosa.
2.10. Data Analyses
The experiment was entirely randomized, with five treatments (four plants and one control), each replicated ten times, with five subsamples and a total of 50 larvae per treatment. The reproductive stage was studied with 10 repetitions, each represented by a cage containing a couple of P. xylostella. Data normality was assessed by the Shapiro–Wilk test. The results were subjected to the Kruskal–Wallis test (p ≤ 0.05). All analyses (2.5, 2.6, 2.7, 2.8 and 2.9) were performed in triplicate, and the results are expressed as mean ± confidence interval (95%). The data were analyzed using the R platform, and p-values lower than 0.05 (p < 0.05) were considered as indicative of significant differences between the samples compared in each test.
3. Results
The aqueous Ludwigia extracts prolonged the larval phase, especially for the L. longifolia extract (χ2 = 16.801; df = 4; p = 0.0021) (Table 1). The percentages of larval survival were lower when all the extracts were used. However, L. tomentosa caused a higher mortality in P. xylostella larvae (χ2 = 10.100; p = 0.0387) (Table 1). Extracts from Ludwigia species prolonged the pupal duration (χ2 = 9.8882; p = 0.0423), pupal survival (χ2 = 562; p = 0.2176) and increased the sex ratio (χ2 = 0.2849; df = 4, p = 0.9908) of P. xylostella (Table 1). We observed that the pupal weights of the larvae treated with L. longifolia (3.87 g) and L. tomentosa (4.58 g) were significantly reduced when compared to the control (5.38 g) (χ2 = 21.202; p = 0.0002) (Table 1).
Table 1.
Duration (days) of and survival (%) during larval and pupal stages, pupal weight (mg) and sex ratio of Plutella. xylostella L. treated with Ludwigia spp. aqueous extracts (25 ± 2 °C; 55 ± 5% relative humidity; 12-h photo phase).
| Treatments | Larval Phase | Pupal Phase | ||||
|---|---|---|---|---|---|---|
| Larval Duration (Days) | Larval Survival (%) | Pupal Duration (Days) | Pupal Survival (%) | Pupal Weight (Mg) | Sex Ratio | |
| Control | 6.54 ± 0.2 b | 88.00 ± 4.4 a | 5.95 ± 0.1 a | 93.50 ± 3.3 a | 5.38 ± 0.1 a | 0.38 ± 0.1 a |
| n = 50 | n = 50 | n = 44 | n = 44 | n = 44 | n = 41 | |
| L. tomentosa | 6.76 ± 0.3 ab | 62.00 ± 7.0 b | 6.47 ± 0.2 a | 88.83 ± 4.7 a | 4.58 ± 0.1 bc | 0.43 ± 0.1 a |
| n = 50 | n = 50 | n = 31 | n = 31 | n = 31 | n = 27 | |
| L. longifolia | 7.72 ± 0.2 a | 72.00 ± 6.8 ab | 6.42 ± 0.1 a | 77.30 ± 8.4 a | 3.87 ± 0.3 c | 0.42 ± 0.1 a |
| n = 50 | n = 50 | n = 35 | n = 35 | n = 35 | n = 27 | |
| L. sericea | 6.56 ± 0.1 b | 80.00 ± 6.0 ab | 6.25 ± 0.1 a | 97.50 ± 2.5 a | 4.92 ± 0.1 ab | 0.39 ± 0.1 a |
| n = 50 | n = 50 | n = 40 | n = 40 | n = 40 | n = 39 | |
| L. nervosa | 7.34 ± 0.3 ab | 86.00 ± 5.2 ab | 6.01±0.1 a | 86.66 ± 4.9 a | 5.11 ± 0.2 ab | 0.45 ± 0.1 a |
| n = 50 | n = 50 | n = 43 | n = 43 | n = 43 | n = 37 | |
| CV (%) | 11.4 | 24.8 | 7.2 | 18.8 | 13.0 | 79.4 |
Means followed by different letters in the same column differ at the 5% significance level when compared using the Tukey test; n = number of individuals. CV—coefficient of variation.
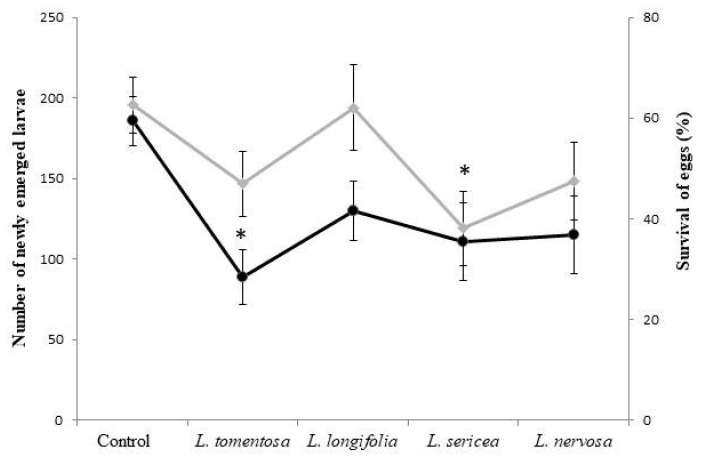
In the adult stage of P. xylostella, the extracts did not significantly influence the longevity of the males (÷2 = 3.5399; p = 0.4718) or females (χ2 = 6.6539; p = 0.1553) or the oviposition period (χ2 = 3.2754; p = 0.5128) (Table 2). We observed a reduction in fertility (÷2 = 19.846; p = 0.0005) (Table 2) and the number of newly emerged P. xylostella larvae (χ2 = 11.421; p = 0.0222) (Figure 3) of P. xylostella for all the treatments. We also noticed a significant reduction with the L. tomentosa extract. Egg survival was reduced by the L. sericea extract (Figure 3).
Table 2.
Longevity of males and females (days), oviposition (days), fecundity and number of newly emerged larvae of Plutella xylostella treated with Ludwigia spp. aqueous extracts (25 ± 2 °C; 55 ± 5% relative humidity; 12-h photo phase).
| Treatments | Longevity of Males (Days) | Longevity of Females (Days) | Oviposition (Days) | Fecundity | Number of Newly Emerged Larvae |
|---|---|---|---|---|---|
| Control | 19.10 ± 1.8 a | 18.60 ± 1.5 a | 13.90 ± 0.8 a | 299.40 ± 13.8 a | 185.60 ± 15.0 a |
| n = 10 | n = 10 | n = 10 | n = 10 | n = 10 | |
| L. tomentosa | 21.70 ± 2.0 a | 13.20 ± 1.5 a | 11.30 ± 1.5 a | 184.40 ± 20.3 b | 88.60 ± 17.0 b |
| n=10 | n = 10 | n = 10 | n = 10 | n = 10 | |
| L. longifolia | 22.20 ± 2.1 a | 18.40 ± 1.7 a | 13.40 ± 1.0 a | 210.90 ± 10.0 ab | 129.80 ± 18.3 ab |
| n = 10 | n = 10 | n = 10 | n = 10 | n = 10 | |
| L. sericea | 22.70 ± 1.8 a | 16.30 ± 1.0 a | 12.30 ± 1.1 a | 297.70 ± 29.3 a | 110. 90 ± 24.2 ab |
| n = 10 | n = 10 | n = 10 | n = 10 | n = 10 | |
| L. nervosa | 17.50 ± 1.9 a | 17.70 ± 1.7 a | 13.70 ± 1.5 a | 229.50 ± 31.6 ab | 115.40 ± 24.1 ab |
| n = 10 | n = 10 | n = 10 | n = 10 | n = 10 | |
| CV (%) | 26.4 | 27.1 | 29.3 | 29.4 | 51.0 |
Means followed by different letters in the same column differ at the 5% significance level when compared using the Tukey test; n = number of individuals. CV—coefficient of variation.
Figure 3.
Number of newly emerged larvae (dark line) and survival of eggs (%) (light line) of Plutella xylostella treated with Ludwigia spp. aqueous extracts. Each data point represents mean ± standard error. (●) Mean number of newly emerged larvae, and (♦) mean percentage of survival of eggs. Asterisks indicate significant differences (* p < 0.05), as determined by the Kruskal–Wallis test.
The antioxidant activity was more prominent for L. longifolia, L. sericea, L. tomentosa and L. nervosa, in that order. Phenolic compounds, flavonoids, condensed tannins and alkaloids were found in greater quantities in the extracts of L. nervosa, L. tomentosa, L. sericea and L. longifolia, in that order (Table 3).
Table 3.
Antioxidant activity data (IC50—minimum inhibitory concentration), phenolic compounds, flavonoids, condensed tannins and alkaloids of species of Ludwigia.
| Aqueous Extract | Antioxidant Activity IC50 (μg/ mL) |
Phenolic Compounds (mg/g) | Flavonoids (mg/g) | Condensed Tannins (mg/g) |
Alkaloids (mg/g) |
|---|---|---|---|---|---|
| Ludwigia tomentosa | 12.8 ± 0.3 | 299.4 ± 0.7 | 162.3 ± 0.9 | 32.5 ± 0.2 | 11.2 ± 0.1 |
| Ludwigia longifolia | 14.9 ± 0.4 | 289.7 ± 0.8 | 144.9 ± 0.8 | 30.9 ± 0.1 | 10.4 ± 0.1 |
| Ludwigia sericea | 13.5 ± 0.2 | 291.5 ± 1.2 | 153.7 ± 1.5 | 31.8 ± 0.1 | 10.7 ± 0.1 |
| Ludwigia nervosa | 9.6 ± 0.1 | 312.4 ± 0.9 | 188.8 ± 1.3 | 34.3 ± 0.2 | 12.5 ± 0.2 |
4. Discussion
The antibiosis effect of Ludwigia extracts was evidenced by the mortality of the individuals in the larval phase and the reduced pupal weight, fecundity, number of newly emerged larvae and percentage of surviving eggs. These phenomena can occur when interfering substances inhibit the food consumption during morphological and physiological transformations, which require intense biochemical activity [44,45,46,47]. The phytochemical screening showed that the Ludwigia species presented all the classes of compounds studied. Our results could be attributed to some of these classes. The prolongation of the larval stage observed during the treatments with the Ludwigia extracts could be due to substances that hinder feeding. These can extend the larval stage due to a lower conversion of ingested aliments [48]. This can occasionally lead to the larvae’s death, principally when using the L. tomentosa extract, as we observed. It could also have caused the reduction in the weights of the pupae when the larvae were treated with the L. tomentosa and L. longifolia extracts.
Studies on herbivores have shown their ability to block leaf consumption, inhibit digestion and, also, create free radicals. The last can rupture the membrane and cause disorders inside insects’ intestinal systems [49], as well as delaying pupal development [50], as observed for all the extracts studied.
Flavonoids are responsible for the reduced growth of larvae [51] and pupal survival [52], impaired feeding, digestion inhibition and the release of free radicals [53]. Flavonoids such as quercetin 3-arabinoside, quercetin 3-glucoside and quercetin 3-rutinoside have already been identified in some species of Ludwigia [54] and found to be able to act as phagodeterrents, depending on the concentrations used [35].
Tannins are another class of compounds with anti-alimental effects [55]. The alkaloids observed may also interfere with neuroendocrine control by inactivating acetylcholinesterase in larvae, causing neurotoxicity [56], in addition to a decrease in weight and increased mortality. This is indicated by a significant decrease in proteins, glycogen, lipids and the activity of the digestive enzyme α-amylase [36].
By interfering with the larvae’s alimentation (without causing death), the compounds can influence the number of ovaries and, therefore, reduce egg production [57]. Studies have shown the biological impact of the flavonoid rutin on the fertility and survival of P. xylostella eggs [22], as well as that of the alkaloid piperine in Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) eggs [58]. This same alkaloid has been found in L. hyssopifolia (G. Don) Exell [59]. The presence of both flavonoids and alkaloids can explain some of the results concerning fertility, the number of hatched eggs and the amount of eggs that survived from the L. tomentosa and L. sericea extracts.
When analyzing each species’ phenolic compounds, flavonoids, condensed tannins, alkaloids and antioxidant activity, statistically significant differences were found (p < 0.05). The species that showed bioactivity in this study were not those that exhibited higher amounts of phenolic compounds, flavonoids, alkaloids and condensed tannins. L. sericea, L. longifolia and L. tomentosa showed greater antioxidant activity, which may be directly linked to the presence of certain flavonoids such as quercetin [60]. However, it is still necessary to keep studying these cases to understand the synergic interactions between compounds.
Among the Ludwigia species presented here, the phytochemical screening of compounds allowed us to use a L. nervosa with a greater quantity of phenolic compounds, flavonoids, tannins and alkaloids. However, the use of the aqueous extract of this plant in P. xylostella did not reduce the number of insects in the tests we performed. Some ideas are raised and should be further studied, such as the use of other solvents, the compounds present and the use of other parts of the plant (stem bark and leaves)—factors that could influence the results obtained [61]. Such findings are well-described in the scientific literature, with studies showing that the aqueous extract of the stem bark of Stryphnodendron adstringens (Mart.) Coville at 10% did not affect the oviposition of P. xylostella [62]. However, the aqueous extract of the leaf at the same concentration inhibited female oviposition [63]. In another study, the methanolic extract of the leaves and stem bark of S. adstringens suppressed oviposition at all tested concentrations, causing a decrease in fertility and the number of newly emerged larvae [28].
5. Conclusions
L. tomentosa, L. longifolia and L. sericea are bioactive species, with L. tomentosa standing out among them. These species showed the best results regarding their ability to control P. xylostella populations, as they influenced important biological characteristics of these insects. Besides that, phytochemical screening showed that the aqueous extracts from these plants contained phenolic compounds, flavonoids, condensed tannins and alkaloids, substances that are able to inhibit food consumption and interfere with the morphological and physiological transformations of the offspring and oviposition of adults. Thus, L. tomentosa, L. longifolia and L. sericea extracts have great potential for use in the control of this pest.
Acknowledgments
The authors thank Mateus Pereira da Silva, Camila Benitez Vilhasanti and Eduardo Carvalho Faca for assistance in collecting the data and Thomás Hernandez and Victória Maria Pompeu Monteiro Padial for reviewing the English. We also thank Sandro Menezes Silva for the identification of the botanical material and Wedson Desidério Fernandes for his scientific writing classes.
Author Contributions
Conceptualization, E.A.F. and R.M.M.; methodology, E.A.F., S.A.d.S., A.D. and R.M.M.; formal analysis, E.A.F., C.A.L.C. and R.M.M.; investigation, E.A.F., M.M.M.D.S. and R.M.M.; resources, E.A.F. and R.M.M.; data curation, E.A.F.; writing—original draft preparation, E.A.F., S.V.d.S., C.A.L.C. and R.M.M.; writing—review and editing, E.A.F., I.M.P.M.P., C.A.L.C., S.V.d.S. and R.M.M.; visualization, E.A.F. and R.M.M.; supervision, E.M.d.C., S.V.d.S. and R.M.M.; project administration, E.A.F. and R.M.M.; funding acquisition, R.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
We would also like to thank the National Council for the Improvement of Higher Education, Brazil (CAPES), for providing a scholarship to the first author, the Foundation for the Development of Education, Science and Technology of Mato Grosso do Sul (FUNDECT) for the resources provided under No. 71/711.130/2018 and the National Council for Scientific and Technological Development (CNPq) (concession number CALC 311975/2018-6).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Furlong M.J., Wright D.J., Dosdall L.M. Diamondback Moth Ecology and Management: Problems, Progress, and Prospects. Annu. Rev. Entomol. 2013;58:517–541. doi: 10.1146/annurev-ento-120811-153605. [DOI] [PubMed] [Google Scholar]
- 2.De Bortoli S.A., Polanczyk R.A., Vacari A.M., Bortoli C.P., Duarte R.T. Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae): Tactics for integrated pest management in Brassicaceae. In: Soloneski S., Larramendy M., editors. Weed and Pest Control—Conventional and New Challenges. Intech; Rijeka, Croatia: 2013. pp. 31–52. [Google Scholar]
- 3.Jaleel W., Saeed S., Naqqash M.N., Sial M.U., Ali M., Zaka S.M., Sarwar Z.M., Ishtiaq M., Qayyum M.A., Aine Q.U., et al. Effects of temperature on baseline susceptibility and stability of insecticide resistance against Plutella xylostella (Lepidoptera: Plutellidae) in the absence of selection pressure. Saudi J. Biol. Sci. 2020;27:1–5. doi: 10.1016/j.sjbs.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammed S., Lamoree M., Ansa-Asare O.D., Boer J. Review of the analysis of insecticide residues and their levels in different matrices in Ghana. Ecotoxicol. Environ. Saf. 2019;171:361–372. doi: 10.1016/j.ecoenv.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Williamson S., Ball A., Pretty J. Trends in pesticide use and drivers for safer pest management in four African countries. Crop Prot. 2008;27:1327–1334. doi: 10.1016/j.cropro.2008.04.006. [DOI] [Google Scholar]
- 6.Pimentel D. Environmental and economic costs of the application of pesticides primarily in the United States. In: Peshin R., Dhawan A.K., editors. Integrated Pest Management: Innovation-Development Process. Volume 1. Springer; Dordrecht, The Netherlands: 2009. pp. 89–111. [Google Scholar]
- 7.Shakeel M., Farooq M., Nasim W., Akram W., Khan F.Z.A., Jaleel W., Zhu X., Yin H., Li S., Fahad S., et al. Environment polluting conventional chemical control compared to an environmentally friendly IPM approach for control of diamondback moth, Plutella xylostella (L.), in China: A review. Environ. Sci. Pollut. Res. 2017;24:14537–14550. doi: 10.1007/s11356-017-8996-3. [DOI] [PubMed] [Google Scholar]
- 8.Navarro-Silva M.A., Marques F.A., Duque L., Jonny E. Review of semiochemicals that mediate the oviposition of mosquitoes: A possible sustainable tool for the control and monitoring of Culicidae. Rev. Bras. Entomol. 2009;53:1–6. doi: 10.1590/S0085-56262009000100002. [DOI] [Google Scholar]
- 9.Abtew A., Subramanian S., Cheseto X., Kreiter S., Garzia G.T., Martin T. Repellency of plant extracts against the legume flower thrips Megalurothrips sjostedti (Thysanoptera: Thripidae) Insects. 2015;6:608–625. doi: 10.3390/insects6030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez-González Á., Álvarez-García S., González-López Ó., Da Silva F., Casquero P.A. Insecticidal Properties of Ocimum basilicum and Cymbopogon winterianus against Acanthoscelides obtectus, Insect Pest of the Common Bean (Phaseolus vulgaris, L.) Insects. 2019;10:151. doi: 10.3390/insects10050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuadrado J.L.C., Pinillos E.O., Tito R., Mirones C.S., Mendoza N.N.G. Insecticidal Properties of Capsaicinoids and Glucosinolates Extracted from Capsicum chinense and Tropaeolum tuberosum. Insects. 2019;10:132. doi: 10.3390/insects10050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayed S.M., Alotaibi S.S., Gaber N., Elarrnaouty S.A. Evaluation of Five Medicinal Plant Extracts on Aphis craccivora (Hemiptera: Aphididae) and Its Predator, Chrysoperla carnea (Neuroptera: Chrysopidae) under Laboratory Conditions. Insects. 2020;11:398. doi: 10.3390/insects11060398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inocente E.A., Nguyen B., Manwill P.K., Benatrehina A., Kweka E., Wu S., Cheng X., Rakotondraibe L.H., Piermarini P.M. Insecticidal and antifeedant activities of Malagasy medicinal plant (Cinnamosma sp.) extracts and drimane-type sesquiterpenes against Aedes aegypti mosquitoes. Insects. 2019;10:373. doi: 10.3390/insects10110373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas P.W., Turner I.M., Dominy N.J., Yamashita N. Mechanical defences to herbivory. Ann. Bot. 2000;86:913–920. doi: 10.1006/anbo.2000.1261. [DOI] [Google Scholar]
- 15.Hagstrum D.W., Aphillips T.W. Evolution of Stored-Product Entomology: Protecting the World Food Supply. Annu. Rev. Entomol. 2017;62:379–397. doi: 10.1146/annurev-ento-031616-035146. [DOI] [PubMed] [Google Scholar]
- 16.Kedia A., Prakash B., Mishra P.K., Singh P., Dubey N.K. Botanicals as eco-friendly biorational alternatives of synthetic pesticides against Callosobruchus spp. (Coleoptera: Bruchidae)—A review. J. Food Sci. Technol. 2015;52:1239–1257. doi: 10.1007/s13197-013-1167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubey N., Shukla R., Kumar A., Singh P., Prakash B. Global scenario on the application of natural products in integrated pest management programmes. Nat. Prod. Plant Pest Manag. 2011;1:1–20. [Google Scholar]
- 18.Amoabeng B.W., Gur G.M., Gitau C.W., Nicol H.I., Munyakazi L., Stevenson P.C. Tri-trophic insecticidal effects of African plants against cabbage pests. PLoS ONE. 2013;8:78651. doi: 10.1371/annotation/f0351003-b6f8-4249-ace5-bcd84dead916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charleston D.S., Gols R., Hordijk K.A., Kfir R., Vet L.E.M., Dicke M. Impact of botanical pesticides derived from Melia azedarach and Azadirachta indica plants on the emission of volatiles that attract parasitoids of the diamondback moth to cabbage plants. J. Chem. Ecol. 2006;32:325–349. doi: 10.1007/s10886-005-9004-9. [DOI] [PubMed] [Google Scholar]
- 20.Charleston D.S., Kfir R., Dicke M., Vet L.E.M. Impact of botanical extracts derived from Melia azedarach and Azadirachta indica on populations of Plutella xylostella and its natural enemies: A field test of laboratory findings. Biol. Control. 2006;39:105–114. doi: 10.1016/j.biocontrol.2006.05.012. [DOI] [Google Scholar]
- 21.Souza S.A., Couto I.F.S., Silva M.P., Cardoso C.A.L., Scalon S.P.Q., Ferreira F.F., Carvalho E.M., Mussury R.M. Aqueous extracts of species of the genus Campomanesia (Myrtaceae) affect biological characteristics of Plutella xylostella (Linnaeus, 1758) Lepidoptera: Plutellidae. J. Agric. Sci. 2019;11:20–28. doi: 10.5539/jas.v11n5p334. [DOI] [Google Scholar]
- 22.Peres L.L.S., Sobreiro A.I., Couto I.F.S., Silva R.M., Pereira F.F., Heredia-Vieira S.C., Cardoso C.A.L., Mauad M., Scalon S.P.Q., Verza S.S., et al. Chemical compounds and bioactivity of aqueous extracts of Alibertia spp. in the control of Plutella xylostella L. (Lepidoptera: Plutellidae) Insects. 2017;8:125. doi: 10.3390/insects8040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amália A.V., Yusa M.H. Control pest of leaf caterpillars (Plutellaxylostella) in delima rose apples using soursop leaf extract (Annona muricata) J. Pendidik. IPA Indones. 2018;7:1–8. doi: 10.15294/jpii.v7i1.12484. [DOI] [Google Scholar]
- 24.Couto I.F.S., Verza S., Valente F.I., Senna B., Souza S.A., Mauad M., Mussury R.M. Botanical Extracts of the Brazilian Savannah Affect Feeding and Oviposition of Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae) J. Agric. Sci. 2019;11:322. doi: 10.5539/jas.v11n5p322. [DOI] [Google Scholar]
- 25.Couto I.F., Fuchs M.L., Pereira F.F., Mauad M., Scalon S.P.Q., Dresch D.M., Mussury R.M. Feeding preference of Plutella xylostella for leaves treated with plant extracts. An. Acad. Bras. Ciênc. 2016;88:1781–1789. doi: 10.1590/0001-3765201620150236. [DOI] [PubMed] [Google Scholar]
- 26.Torres A.L., Boiça Júnior A.L., Medeiros C.A.M., Barros R. Efeito de extratos aquosos de Azadirachta indica, Melia azedarach e Aspidosperma pyrifolium no desenvolvimento e oviposição de Plutella xylostella. Bragantia. 2006;65:447–457. doi: 10.1590/S0006-87052006000300011. [DOI] [Google Scholar]
- 27.Trindade R.C.P., Araújo Junior J.X., Sant’Ana A.E.G., Aquino P.G.V., Silva Sousa R., Araújo Costa A.P.A. Utilização de extratos aquosos de Aspidosperma macrocarpum sobre diferentes estágios de lagartas da traça-das-crucíferas, em couve. Rev. Ciênc. Agric. 2014;12:21–26. doi: 10.28998/rca.v12i1.1083. [DOI] [Google Scholar]
- 28.Fonseca J., Couto I.F.S., Matias R.S., Fioratti C.A.G., Pereira F.F., Mauad M., Scalon S.P.Q., Carvalho E.M., Mussury R.M. Efeito de extratosmetanólicos de Stryphnodendron adstringens (Mart.) Coville na alimentação e reprodução de Plutella xylostella L. (Lepidoptera: Plutellidae) Interciencia. 2018;43:182–187. [Google Scholar]
- 29.Fouad H.A., Camara C.A.G. Chemical composition and bioactivity of peel oils from Citrus aurantiifolia and Citrus reticulata and enantiomers of their major constituent against Sitophilus zeamais (Coleoptera: Curculionidae) J. Stored Prod. Res. 2017;73:30–36. doi: 10.1016/j.jspr.2017.06.001. [DOI] [Google Scholar]
- 30.Chai T.T., Ooh K.F., Quah Y., Wong F.C. Edible freshwater macrophytes: A source of anticancer and antioxidative natural products—A mini-review. Phytochem. Rev. 2015;14:443–457. doi: 10.1007/s11101-015-9399-z. [DOI] [Google Scholar]
- 31.Mabou F.D., Jean D.D.T., Ngnokam D., Voutquenne-Nazabadioko L., Kuiate J.R., Bag P.K. Complex secondary metabolites from Ludwigia leptocarpa with potent antibacterial and antioxidant activities. Drug Discov. Ther. 2016;10:141–149. doi: 10.5582/ddt.2016.01040. [DOI] [PubMed] [Google Scholar]
- 32.Kadum Yakob H., Manaf Uyub A., Fariza Sulaiman S. Toxicological evaluation of 80% methanol extract of Ludwigia octovalvis (Jacq.) P. H. Raven leaves (Onagraceae) in BALB/c mice. J. Ethnopharmacol. 2012;142:663–668. doi: 10.1016/j.jep.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed F., Selim M.S.T., Shilpi J.A. Antibacterial activity of Ludwigia adscendens. Fitoterapia. 2005;76:473–475. doi: 10.1016/j.fitote.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Oyedeji O., Oziegbe M., Taiwo F.O. Antibacterial, antifungal and phytochemical analysis of crude extracts from the leaves of Ludwigia abyssinica A. Rich. and Ludwigia decurrens Walter. J. Med. Plants Res. 2011;5:1192–1199. [Google Scholar]
- 35.Diaz Napal G.N., Defago M.T., Valladares G.R., Palacios S.M. Response of Epilachnapaenulata to two flavonoids, pinocembrin and quercetin, in a comparative study. J. Chem. Ecol. 2010;36:898–904. doi: 10.1007/s10886-010-9823-1. [DOI] [PubMed] [Google Scholar]
- 36.Bouayad N., Rharrabe K., Lamhamdi M., Nourouti N.G., Sayah F. Dietary effects of harmine, a β-carboline alkaloid, on development, energy reserves and α-amylase activity of Plodia interpunctella Hübner (Lepidoptera: Pyralidae) Saudi J. Biol. Sci. 2012;19:73–80. doi: 10.1016/j.sjbs.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Procópio T.F., Fernandes K.M., Pontual E.V., Ximenes R.M., Oliveira A.R.C., Santana Souza C., Melo A.M.M.A., Navarro D.M.A.F., Paiva P.M.G., Martins G.F., et al. Schinus terebinthifolius leaf extract causes midgut damage, interfering with survival and development of Aedes aegypti larvae. PLoS ONE. 2015;10:e0126612. doi: 10.1371/journal.pone.0126612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barros R., Thuler R.T., Pereira F.F. Técnica de criação de Plutella xylostella (L. 1758) (Lepidoptera: Yponomeutidae) In: Pratissoli D., editor. Técnicas de Criação de Pragas de Importância Agrícola, em Dietas Naturais. 1st ed. Edufes; Vitória, Brasil: 2012. pp. 65–84. [Google Scholar]
- 39.Matias da Silva R., Fioratti C.A.G., Silva G.B., Cardoso C.A.L., Miranda L.O., Mauad M., Mussury R.M. Antibiose do extrato foliar de Duguetia furfuracea sobre Plutella xylostella (Lepidoptera: Plutellidae) In: Calixto E.S., Toreza-Silingardi H.M., editors. Temas Atuais em Ecologia Comportamental e Interações. Anais do II BecInt—Behavioral Ecology and Interactions Symposium. 1st ed. Volume 1. Editora Composer; Uberlândia, Brasil: 2017. pp. 52–69. (In Portuguese) [Google Scholar]
- 40.Djeridane A., Yousfi M., Nadjemi B., Boutassouna D., Stocker P., Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. doi: 10.1016/j.foodchem.2005.04.028. [DOI] [Google Scholar]
- 41.Broadhurst R.B., Jones W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978;29:788–794. doi: 10.1002/jsfa.2740290908. [DOI] [Google Scholar]
- 42.Oliveira M.A.C., Albuquerque M.M., Xavier H.S., Strattmann R.R., GrangeiroJúnior S., Queiroz A.T. Development and validation of a method for the quantification of total alkaloids as berberine in an herbal medicine containing Berberis vulgaris L. Rev. Bras. Farmacogn. 2006;16:357–364. doi: 10.1590/S0102-695X2006000300013. [DOI] [Google Scholar]
- 43.Kumaran A., Karunakaranm R.J. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006;97:109–114. doi: 10.1016/j.foodchem.2005.03.032. [DOI] [Google Scholar]
- 44.Duffey S.S., Isman M.B. Inhibition of insect larval growth by phenolics in glandular trichomes of tomato leaves. Experientia. 1981;37:574–576. doi: 10.1007/BF01990057. [DOI] [Google Scholar]
- 45.Isman M.B., Duffey S.S. (Toxicity of tomato phenolic compounds to the fruitworm, Heliothiszea. Entomol. Exp. Appl. 1982;31:370–376. doi: 10.1111/j.1570-7458.1982.tb03162.x. [DOI] [Google Scholar]
- 46.Summers C.B., Felton G.W. Pro oxidant effects of phenolic acids on the generalis herbivore Helicoverpa zea (Lepidoptera: Noctuidae): Potential modeo faction for phenolic compounds in plantanti-herbivore chemistry. Insect Biochem. Mol. Biol. 1994;24:943–953. doi: 10.1016/0965-1748(94)90023-X. [DOI] [Google Scholar]
- 47.Pan L., Ren L., Chen F., Feng Y., Luo Y. Antifeedant activity of Ginkgo Biloba secondary metabolites against Hyphantria cunea larvae: Mechanisms and applications. PLoS ONE. 2016;11:155682. doi: 10.1371/journal.pone.0155682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanzubil P.B., Mccaffery A.R. Effects of azadirachtin and aqueous neem seed extracts on survival, growth and development of the African armyworm, Spodoptera exempta. Crop Prot. 1990;9:383–386. doi: 10.1016/0261-2194(90)90012-V. [DOI] [Google Scholar]
- 49.Baldin E.L.L., Vendramim J.D., Lourenção A.L. Resistência de Plantas a Insetos: Fundamentos e Aplicações. FEALQ; Piracicaba, Brazil: 2019. [Google Scholar]
- 50.Aluja M., Birkea A., Ceymannb M., Guilléna L., Arrigonib E., Baumgartnerb D., Pascacio-Villafána C., Samietzb J. Agroecosystem resilience to an invasive insect species that could expand its geographical range in response to global climate change. Agric. Ecosyst. Environ. 2014;186:54–63. doi: 10.1016/j.agee.2014.01.017. [DOI] [Google Scholar]
- 51.Stamp N.E., Skrobola C.M. Failure to avoid rutin diets results in altered food utilization and reduced growth rate of Manduca sexta larvae. Entomol. Exp. Appl. 1993;68:127–142. doi: 10.1111/j.1570-7458.1993.tb01696.x. [DOI] [Google Scholar]
- 52.Silva T.R.F.B., Almeida A.C.S., Moura T.L., Silva A.R., Freitas S.S., Jesus F.G. Effect of the flavonoid rutin on the biology of Spodoptera frugiperda (Lepidoptera: Noctuidae) Acta Sci. Agron. 2016;38:165–170. doi: 10.4025/actasciagron.v38i2.27956. [DOI] [Google Scholar]
- 53.Appel H.M. Phenolics in ecological interactions: The importance of oxidation. J. Chem. Ecol. 1993;19:1521–1552. doi: 10.1007/BF00984895. [DOI] [PubMed] [Google Scholar]
- 54.Averett J.E., Zardini E.M., Hoch P.C. Flavonoid systematics of ten sections of Ludwigia (Onagraceae) Biochem. Syst. Ecol. 1990;18:529–532. doi: 10.1016/0305-1978(90)90124-X. [DOI] [Google Scholar]
- 55.Lago J.H.G., Brochini C.B., Roque N.F. Terpenoids from Guarea guidonia. Phytochemistry. 2002;60:333–338. doi: 10.1016/S0031-9422(02)00089-4. [DOI] [PubMed] [Google Scholar]
- 56.Acheuk F., Doumandji-Mitiche B. Insecticidal activity of alkaloids extract of Pergularia tomentosa (Asclepiadaceae) against fifth instar larvae of Locusta migratoria cinerascens (Fabricius 1781) (Orthoptera: Acrididae) Int. J. Mol. Sci. 2013;3:8–13. [Google Scholar]
- 57.Costa E.L., Silva N.R.F.P., Fiúza L.M. Efeitos, aplicações e limitações de extratos de plantas inseticidas. Acta Biol. Leopold. 2004;26:173–185. [Google Scholar]
- 58.Tavares W.S., Cruz I., Petacci F., Freitas S.S., Serratilde J.E., Zanuncio J.C. Insecticide activity of piperine: Toxicity to eggs of Spodoptera frugiperda (Lepidoptera: Noctuidae) and Diatraea saccharalis (Lepidoptera: Pyralidae) and phytotoxicity on several vegetables. J. Med. Plants Res. 2011;5:5301–5306. [Google Scholar]
- 59.Das B., Kundu J., Bachar S.C., Uddin M.A., Kundu J.K. Antitumor and antibacterial activity of ethylacetate extract of Ludwigia hyssopifolia linn and its active principle piperine. Pak. J. Pharm. Sci. 2007;20:128–131. [PubMed] [Google Scholar]
- 60.Xu D., Hu M.J., Wang Y.Q., Cui Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24:1123. doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roel A.R., Vendramim J.D., Frighetto R.T., Frighetto N. Atividade tóxica de extratos orgânicos de Trichilia pallida Swartz (Meliaceae) sobre Spodoptera frugiperda (J.E. Smith) An. Soc. Entomol. Bras. 2000;29:799–808. doi: 10.1590/S0301-80592000000400021. [DOI] [Google Scholar]
- 62.Medeiros C.A.M., Boica A.L., Jr., Leite A. L Efeito de extratos aquosos de plantas na oviposição da traça-das-crucíferas, em couve. Bragantia. 2005;64:227–232. doi: 10.1590/S0006-87052005000200009. [DOI] [Google Scholar]
- 63.Jesus F.G., Paiva L.A., Gonçalves V.C., Marques M.A., Boiça A., Jr. Efeito de plantas inseticidas no comportamento e biologia de Plutella xylostella (Lepidoptera: Plutellidae) Arq. Inst. Biol. São Paulo. 2011;78:279–285. [Google Scholar]