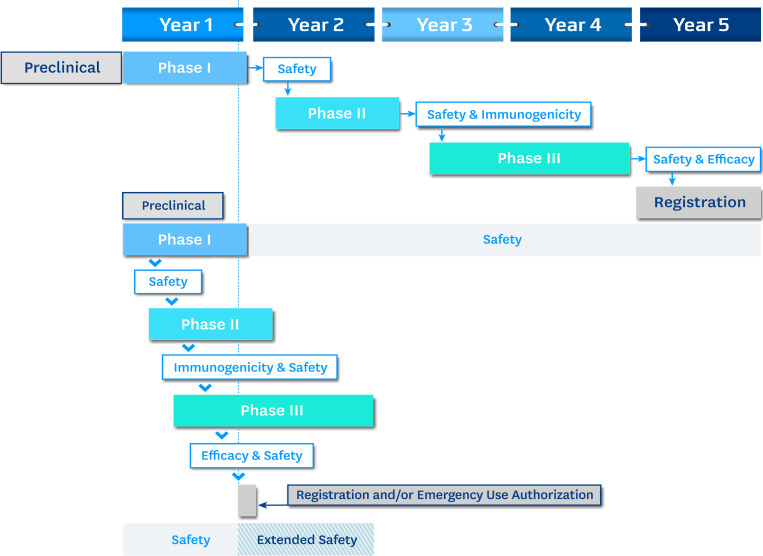
Figure 1.
Schematic of hypothetical 5-yr and 1-yr vaccine development timelines. Phase I human trials often have ~50 volunteers; Phase II trials have 200–500 volunteers; and Phase III trials involve thousands (often tens of thousands) of volunteers. As seen in the 1-yr development cycle, it is possible to telescope the phases, and it is further possible (subject to regulatory review) to combine clinical trial phases (Phase I/II or Phase II/III). Safety and efficacy remain the key elements in any licensure or emergency use decision.