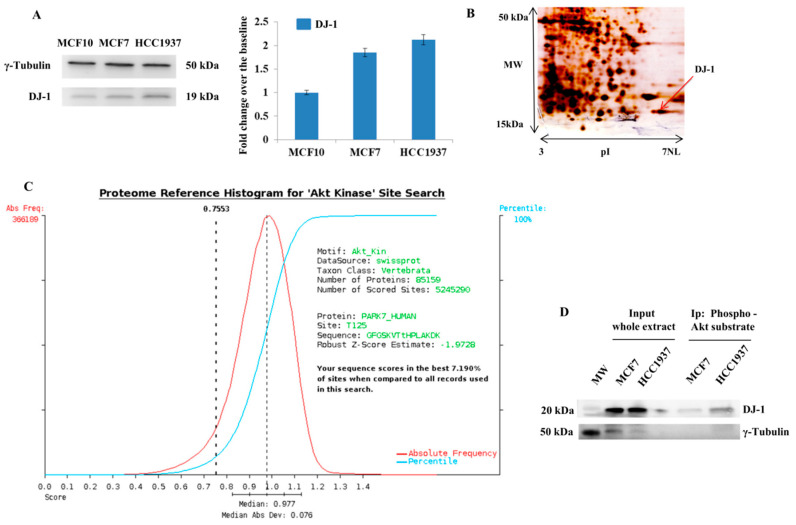
Figure 2.
Identification of Novel Phosphorylation on DJ-1 (A) Western blot and densitometry analysis of DJ-1 in whole protein extracts from MCF10, MCF7, and HCC1937 cells. Whole protein extracts were resolved using AnykD precast gels and blotted on nitrocellulose membrane using turbo blot. Filter were hybridized with primary antibody against DJ-1 (D29E5XP, Cell Signaling; Danvers, MA, USA). Equal loading of proteins was confirmed using Anti-γ-Tubulin antibody (C-20, Santa Cruz). Each assay was repeated in three independent biological replicates. The histogram shows the level of DJ-1, expressed as fold change over the baseline, in breast cancer cells (MCF7 and HCC1937) compared to normal immortalized breast cells (MCF10); (B) 2D gel map of MCF7 breast cancer cells. Protein spot identified as DJ-1, through LC-MS/MS analysis, is indicated with a black arrow; (C) scansite results. The scansite search on DJ-1 allows us to detect that the Phospho-peptide VTtHPLAK, identified by LC-MS/MS analysis, encloses a putative Akt consensus; (D) The ability of Akt to directly interact with DJ-1 was investigated by immunoprecipitation experiments. We incubated the protein extracts from MCF7 and HCC1937 breast cancer cells using Sepharose Bead Conjugate with Phospho-Akt Substrate (RXXS*/T*) antibody. The input and the immunocomplexes, containing isolated Akt substrates, were resolved by SDS gel and blotted onto nitrocellulose membrane. The membrane was assayed with antibodies against DJ-1. γ-Tubulin was used as loading control.