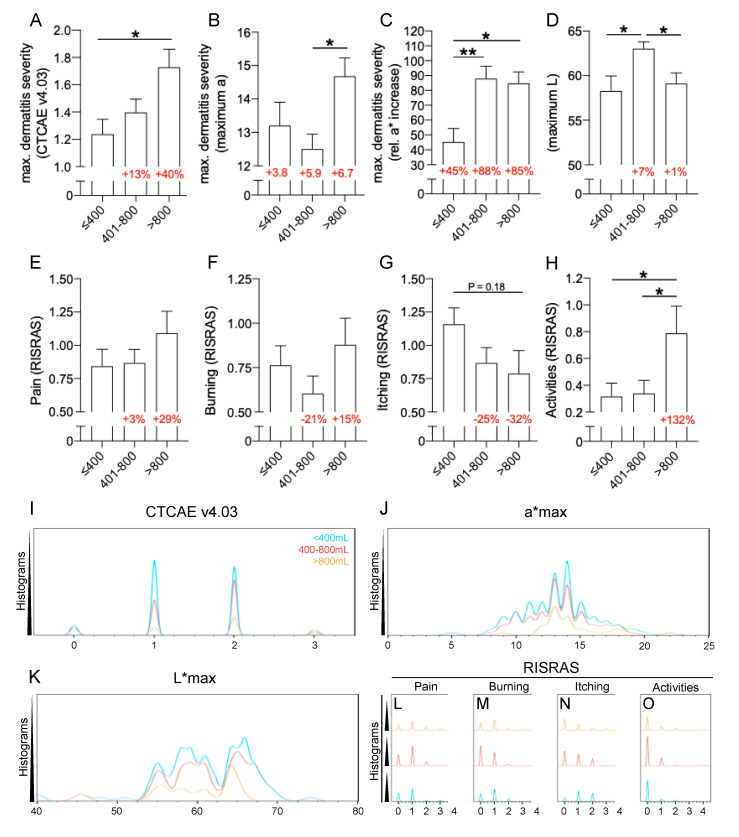
Figure 2.
Breast volume is a positive risk factor for severe RID. (A) Maximum dermatitis severity was assessed by physicians according to CTCAE v4.03 in relation to different breast volumes. Percentages in red indicate the relative increase to the dermatitis severity for patients with a breast volume of <400 mL. (B) Maximum dermatitis severity is measured by spectrophotometry as maximum a* values in relation to different breast volumes. Numbers in red indicate the absolute increase to baseline a* values. (C) Maximum dermatitis severity is measured by spectrophotometry as relative a* value-increase (maximum to baseline) in relation to different breast volumes. Numbers in red indicate the relative increase to baseline a* values. (D) The lightness of the skin during RID enumerated by absolute L* maximum values obtained by SP. Red numbers indicate the alteration relative to patients with small breasts. (E–H) Subjective RISRAS assessment of RID related symptoms. (E) Pain (F) Burning (G) Itching (H) Reduction in everyday life activities. Red numbers indicate the alteration relative to patients with small breasts. (I–O) Distribution pattern analyses according to breast size <400 mL (light blue), 400–800 mL (red) and >800 mL (orange). (I) Visual CTCAE v4.03 grading (J) Maximum a* value (K) Maximum L* values (L) Pain assessed by RISRAS. (M) Burning assessed by RISRAS (N) Itching assessed by RISRAS (O) Reduction in everyday life activities. One-way-ANOVA has been conducted for significance detection with * for p < 0.05, ** for p < 0.01.