Figure 2.
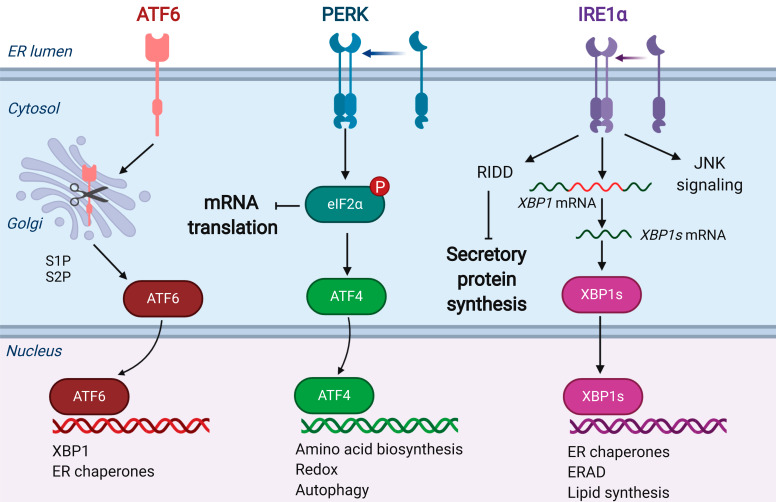
Regulation of ER Stress by the Unfolded Protein Response and Effects on Cardiokine Secretion. Perturbations of the ER environment result in the activation of the unfolded protein response, mediated by ER-resident integral membrane proteins ATF6 (activating transcription factor 6), PERK (protein kinase RNA (PKR)-like ER kinase), and IRE1α (inositol requiring enzyme 1). Upon ER stress, ATF6 translocates to the Golgi apparatus, where it is cleaved by site-1 and site-2 proteases (S1P, S2P), liberating an N-terminal fragment to translocate to the nucleus, where it is a potent and labile transcription factor that activates the transcription of ER chaperones and the transcription factor X-box binding protein 1 (XBP1). Activation of ATF6 may increase ER secretory capacity [35]. PERK dimerization activates autophosphorylation of its kinase domain, leading to phosphorylation of the α-subunit of translation initiation factor 2 (eIF2α) at Ser51. This inhibits global translational initiation, including translation of mRNAs encoding secretory proteins, reducing the load of unfolded proteins entering the ER. PERK activation also results in the induction of translation of selective mRNAs, including activating transcription factor 4 (ATF4), which activates the transcription of a wide range of genes involved in adaptation to stress conditions. Upon activation, IRE1α oligomerizes and carries out regulated IRE1-dependent decay (RIDD) of ER-targeted transcripts, as well as unconventional RNA splicing, by removing an intron from the XBP1 mRNA, allowing it to become translated into the functional transcription factor, XBP1s. XBP1s activates the transcription of ER chaperones, endoplasmic reticulum associated degradation (ERAD) genes, and genes involved in lipid synthesis.