Abstract
Ganoderma lucidum is a popular traditional Chinese medicine used in China to improve health. Previous researches have revealed that the polysaccharide from G. lucidum could exert diversity activities, including immunomodulation, antioxidant, and antitumor effects. However, the effect of enzymatically hydrolyzed G. lucidum polysaccharide (EGLP) in colorectal cancer (CRC) progression remains unknown. The present research aimed to investigate the antitumor mechanism of EGLP in human colon cancer cells. For this purpose, the cytotoxic effects of EGLP were measured by the (3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide (MTT) method. The apoptosis was evoked upon EGLP treatment, which was assayed using flow cytometry. The results indicated that EGLP may induce apoptosis in human colon cancer cell (HCT-116) cells via the upregulation of BCL-2 associated X protein (Bax), phospho-extracellular regulated protein kinases (P-ERK), and cleaved caspase-3 expression and downregulation of B-cell lymphoma-2 (Bcl-2), phospho-serine/threonine kinase 1 (p-Akt1), and cyclo-oxygen-ase (COX-2) expression. The obtained findings indicated EGLP as a new therapeutic agent in fighting CRC.
Keywords: colon cancer, enzymatically hydrolyzed polysaccharide, Ganoderma lucidum
Introduction
Colorectal cancer (CRC) is the third most deadly cancer for men and women and the third most frequently diagnosed cancer across the world1. This prevalence has turned CRC into a major health problem across the world, and its incidence is promptly rising in China. Although research continues for more effective therapies, surgery, chemotherapy, and radiotherapy are the most commonly used treatments of CRC. However, organ toxicity, eventual resistance, and limited effectiveness remain serious problems2. Thus, novel therapeutic strategies are urgently required to assuage those side effects and enhance antitumor activity associated with this malignancy. However, it is well established that lifestyles and environmental factors, including physical inactivity, smoking, diet low in fruits or vegetables along with diet rich in red meat and saturated fat, are involved in the development of CRC3,4. In addition, many researches imply that diet habits play an important role in CRC carcinogenesis5. Thus, dietary regulation by consumption of natural nutrients or pharmacological agents with cancer chemopreventive effects could decrease the incidence of CRC6. Natural polysaccharides are the main biologically active components in natural medicine7.
Ganoderma lucidum is a popular fungus and has been widely used as a traditional Chinese medicine in China for the prevention and treatment of a variety of diseases. Polysaccharides are the main active components of G. lucidum and exert a wide range of pharmacological activities, including immunomodulation, anticolon carcinoma, and antioxidation effects8,9. The pharmacological activities of polysaccharides are closely related to their molecular structures, including sulfate content, viscosity, average molecular weight, and monosaccharide proportion10. High molecular weights, structural complexity, and viscous properties of crude polysaccharides may hinder their clinical application, particularly as therapeutic agents11. Low molecular weight polysaccharide could overcome these problems. Previous researches have reported that enzymatically degraded polysaccharide possesses higher antioxidative, antimelanogenesis, and moisture-preserving activities than the crude polysaccharide10,12. Besides, increasing evidence has shown that low molecular weight polysaccharide exerts anticancer effects toward different tumors, such as ovarian cancer, colon cancer, and prostate cancer13. Thus, the production of low molecular weight oligosaccharide from G. lucidum is necessary to improve its biological activity.
According to the literature, there are several chemical and physical ways to hydrolyze the crude polysaccharide, such as oxidative degradation, ultrasonic degradation, and enzymatic degradation14,15. Among them, enzymatic degradation is an effective way for high selectivity and substrate specificity, which could produce a well-defined structure of polysaccharide10. As far as we know, little research on the anticolon cancer effect of the enzymatically hydrolyzed G. lucidum polysaccharide (EGLP) has been conducted. In the present research, the effect of EGLP on colorectal cancer cell apoptosis in vitro was further investigated and the underlying mechanism of its action was investigated.
Materials and Methods
Materials and Chemicals
The fruiting body of G. lucidum was procured from Xunwu Hengshun biotechnology company (Ganzhou, Jiangxi, China) and was identified as an artificial cultivar. Cellulase was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other solvents and chemicals used were procured from Aladdin (Shanghai, China).
Extraction and Separation of Crude G. lucidum Polysaccharide
G. lucidum polysaccharide (GLP) was extracted from the fruiting body of G. lucidum by hot water extraction based on previous literature with minor modification16. Briefly, the extraction parameters were extraction temperature of 95°C, extraction time of 2 h, and water/solid of 12:1. After the extract was centrifuged, the supernatant was collected and condensed at 60°C under vacuum, and then added into four volumes of 70% ethanol at 2°C for 12 h to precipitate the polysaccharide. To remove the proteins, the precipitates were deproteinated by Sevag method17. Then the crude polysaccharide was further purified with dialysis membrane (cutoff 10 kDa) against pure water. Finally, the high molecular weight crude polysaccharide was dried by lyophilization and named GLP.
Preparation of GLP-Degraded Fragments
GLP was enzymatically degraded by cellulase for 2 h under the condition of pH 5.5 and 50°C. The hydrolysate was dialyzed with dialysis membrane (cutoff 10 kDa) against pure water. The nondialysate was collected and dried by lyophilization. The degradation fragment from hydrolysates of 2 h was named EGLP. The degradation fragment was further purified with the dialysis membrane (cutoff 10 kDa) against pure water. Finally, EGLP was dried by lyophilization. The content of carbohydrate was measured by the phenol–sulfuric acid method. Sulfate content was measured by barium chloride–gelatin method. Protein content was measured by the Bradford method. Uronic acid content was measured by uronic acid carbazole method.
Molecular Weight and Homogeneity Analysis by SEC-MALLS-RI Measurement
The molecular weight and homogeneity of GLP/EGLP were assayed by size exclusion chromatography combined with multiangle laser light scattering detector and refractive index detector (SEC-MALLS-RI, Waters 2695, USA). Shodex-OHpak SB-804 HQ column (Shodex, Japan) (8.0 mm × 300 mm) was used as the separation medium. The mobile phase was 0.1 M sodium chloride at a flow rate of 0.7 ml/min. The column temperature was set at 30°C, all samples were dissolved with the mobile phase, and the injection volume was 0.1 ml (1 mg/ml).
1H-NMR Analysis
EGLP, 30.0 mg, was weighted and dissolved in D2O (0.5 ml). Nuclear magnetic resonance (1H-NMR, Zhongke-Niuji) of the EGLP was measured by AS 400 MHz NMR spectrometer (Wuhan, China).
Cell Lines and Cell Culture
Human colorectal adenocarcinoma HCT-116 and colon epithelial nontumorigenic (CCD18-Co) cell lines were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were grown in Roswell Park Memorial Institute (RPMI-1640) medium (Thermo Fisher), and culture media were supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin (Sigma-Aldrich), and 10% fetal bovine serum (Thermo Fisher), and maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Cell Viability Assay
The effect of GLP or EGLP on HCT-116 and CCD18-Co cells survival was measured by MTT assay according to the previous method with some modifications18. Briefly, 5 × 104 cells/well of colon cancer or CCD18-Co cells were cultured in a 96-well plate for 24 h with 5% CO2 at 37°C. The cells were treated with 150 µg/ml (GLP/EGLP) or an equivalent volume of dimethyl sulfoxide (DMSO) vehicle control for 24, 48, and 72 h. After the indicated incubation time, 20 µl of MTT reagent was added into each well. After incubation for 2 h, the media was removed, and the formed insoluble formazan crystals were dissolved in DMSO and quantified at 540 nm using the microplate reader (BioTek, Winooski, VT, USA). All samples were assayed in quadruplicate and experiments were repeated three times.
Cell Apoptotic Analysis
The Annexin fluorescein isothiocyanate (V-FITC) apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to measure HCT-116 cell apoptosis according to the manufacturer’s protocol. Briefly, the HCT-116 cells were seeded in complete culture medium and incubated with EGLP (150 and 300 µg/ml) for 48 h. Then, the HCT-116 cells were collected by centrifugation at 10,000 rpm for 5 min at 4°C and resuspended in 500 µl Annexin V-FITC binding buffer. HCT-116 cells were incubated with 5 µl Annexin V-FITC and 5 µl propidium iodide (PI) in the dark for 15 min. The apoptosis rates were quantitatively analyzed with a FACSCalibur flow cytometer (Beckman Coulter, Brea, CA, USA).
Western Blot Analysis
HCT-116 cells were collected and lysed in radio immunoprecipitation assay (RIPA) lysis buffer (Sigma-Aldrich) containing protease inhibitors and phosphatase. Protein concentration of the lysates was quantified using the bicinchoninic acid Protein Assay Kit (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). The proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 110 V for 2 h and then transferred onto a polyvinylidene fluoride membrane (Millipore, Boston, MA, USA) at 60 V for 2 h. After blocking with 5% nonfat milk at room temperature for 1 h, the polyvinylidene fluoride membranes were incubated with anti-Bcl-2 (1:1,000; Abcam, Cambridge, UK), anti-Bax (1:1,000; Abcam), anti-p-Akt1 (1:1,000; Abcam), anti-P-ERK (1:1,000; Abcam), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1,000; Abcam) primary antibodies overnight at 4°C. The polyvinylidene fluoride membranes were washed with tris buffered saline tween (TBST) and incubated with a horseradish peroxidase–conjugated secondary antibody at room temperature for 45 min. Visualization was performed with a chemiluminescent detection kit (Millipore). The densitometry of the bands was analyzed using Gel-Pro 6.3 software (Media Cybernetics, Inc., Bethesda, MD,USA).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total ribonucleic acid (RNA) was isolated from HCT-116 cells using Trizol reagent (Takara Bio, Inc., Dalian, Liaoning, China) according to the manufacturer’s protocol. Complementary deoxyribonucleic acid (cDNA) was synthesized from total RNA using the First Strand cDNA Synthesis Kit (TransGen, Beijing, China). RT-PCR was carried out with an SYBR Green qPCR Master Mix kit (Takara Bio, Inc.). The procedure of RT-PCR amplification reaction is as follows: 40 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 15 s with the primer sequences (Table 1), and GAPDH acted as an internal control. The relative messenger ribonucleic acid (mRNA) expression was calculated by the 2ΔΔCT method.
Table 1.
Sequences of Primers Used in Quantitative Real-Time Polymerase Chain Reaction.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Bax | 5′-GGCCCTTTTGCTTCAGGGTT-3′ | 5′-GGAAAAAGACCTCTCGGGGG-3′ |
| Bcl-2 | 5′-CTTTGAGTTCGGTGGGGTCA-3′ | 5′-GGGCCGTACAGTTCCACAAA-3′ |
| COX-2 | 5′-TTTGCATTCTTTGCCCGC-3′ | 5′-GGGAGGATACATCTCTCCATCAAT-3′ |
| Cleaved caspase-3 | 5′-AGCAATAAATGAATGGGCTGAG-3′ | 5′-GTATGGAGAAATGGGCTGTAGG-3′ |
| GAPDH | 5′-GAACGGGAAGCTCACTGGC-3′ | 5′-GCATGTCAGATCCACAACGG-3′ |
Bax: BCL-2 associated X protein; Bcl-2: B-cell lymphoma-2; COX-2: cyclo-oxygen-ase-2; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Statistical Analysis
All experiments were carried out three times and the results were expressed as mean ± SD. All results obtained were analyzed using a one-way analysis of variance followed by Tukey’s multiple comparison test. Values with P < 0.05 were used to determine significant differences.
Results
Chemical Analysis of GLP and Its Degraded Fragments
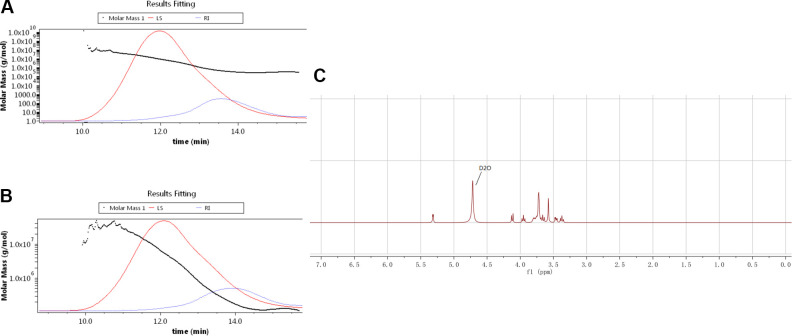
As displayed in Table 2, comparison of enzymatic degradation fractions showed no obvious difference existed but in molecular weight. The total polysaccharides contents of GLP and EGLP assayed by the phenol–sulfuric acid method were 88.63% and 89.16%, respectively. Meanwhile, the molecular weights of GLP and EGLP were determined by SEC-MALLS-RI (Fig. 1A, B). The molecular weight of GLP was 1,626 kDa, which was higher than EGLP (816.1 kDa). These results indicated that GLP was enzymatically degraded into lower molecular weight polysaccharide. In addition, Fig. 1C shows the 1H-NMR of EGLP. The typical peak distribution of polysaccharides was at δH 3.0–5.5 ppm.
Table 2.
The Chemical Property of Polysaccharide Fractions from Ganoderma lucidum.
| Samples | GLP | EGLP |
|---|---|---|
| Mw (kDa) | 1,626 | 816.1 |
| Polydispersity (Mw/Mn) | 3.65 | 4.06 |
| Total polysaccharide (%) | 88.63 ± 1.82 | 89.16 ± 1.49 |
| Protein (%) | 0.61 ± 0.02 | 0.47 ± 0.03 |
| Uronic acids (%) | 2.05 ± 0.11 | 2.62 ± 0.09 |
| Sulfate (%) | 3.14 ± 0.05 | 6.27 ± 0.06 |
EGLP: enzymatically hydrolyzed G. lucidum polysaccharide; GLP: G. lucidum polysaccharide; Mw: xxx; Mn: xxx.
Fig. 1.
The molar mass, RI, and LS chromatograms for GLP (A) and EGLP (B). 1H-NMR spectra of EGLP (C). EGLP: enzymatically hydrolyzed G. lucidum polysaccharide; GLP: G. lucidum polysaccharide; LS: xxx; NMR: xxx; RI: xxx.
Effects of EGLP on the Viability of HCT-116 and CCD18-Co Cells
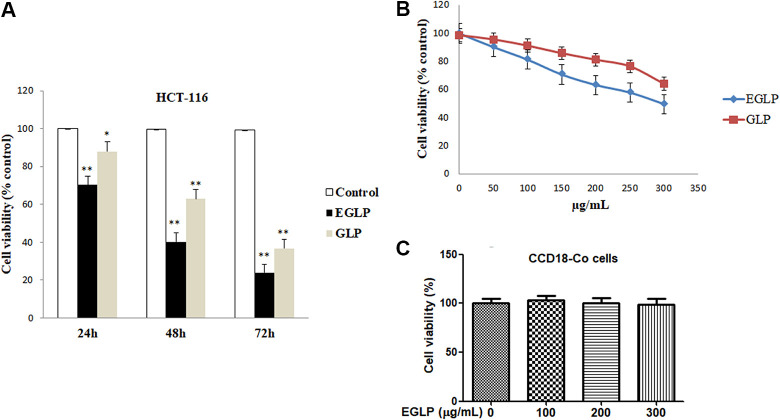
The colon cancer cytotoxicity induced by GLP or EGLP was investigated in human colon cancer cells (HCT-116) using the MTT assay. As displayed in Fig. 2A, a time-dependent reduction in colon cancer cell viability was observed following treatment with the same concentration of GLP or EGLP in HCT-116 cells. Additionally, as shown in Fig. 2B, the proliferation of HCT-116 cells was dose-dependently suppressed by 50, 100, 150, 200, 250, and 300 µg/ml GLP or EGLP. Interestingly, the HCT-116 cells inhibitory effects of GLP were always lower than those of the enzymatic hydrolysates at the same incubation time. The results revealed that enzymatic degradation fractions of GLP exhibited more effective colon cancer cells inhibitory effect than did the intact GLP. This finding was consistent with the previous researches that the low molecular weight of degradation polysaccharides exerted stronger anticolon activity than the native polysaccharides13,19. To further investigate the effect of EGLP on the viability of normal cell line, CCD18-Co cells were treated with EGLP at different doses. As shown in Fig. 2C, the viability of CCD18-Co cells was not suppressed after treatment with EGLP. The findings showed that EGLP inhibited the viability of HCT-116 but not that of the normal cells in a concentration-dependent manner.
Fig. 2.
Inhibitory effects of native (GLP) and enzymatically modified (EGLP) polysaccharides from Ganoderma lucidum against the formation of colon cancer cells HCT-116. (A) HCT-116 cells were treated with EGLP or GLP (150 µg/ml) for 24, 48, and 72 h. (B) HCT-116 cells were treated with EGLP or GLP at different concentrations (0, 50, 100, 150, 200, 250, and 300 µg/ml) for 24 h. (C) CCD18-Co cells were treated with EGLP at different concentrations (0, 100, 200, and 300 µg/ml) for 24 h. The data are reported as mean ± SD (three independent experiments). **P < 0.01 (vs. the control group), *P < 0.05 (vs. the control group). CCD18-Co: xxx; EGLP: enzymatically hydrolyzed G. lucidum polysaccharide; GLP: G. lucidum polysaccharide; HCT-116: xxx.
EGLP Induces the Apoptosis of HCT-116 Cells
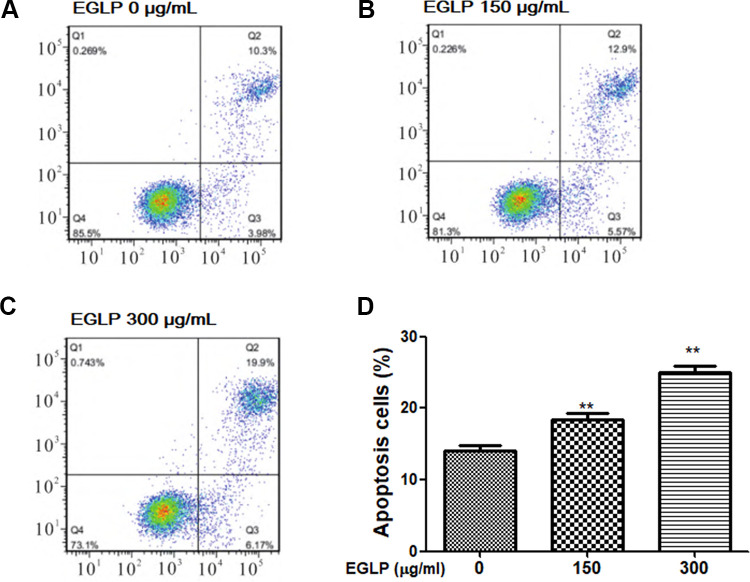
The effects of EGLP on HCT-116 cells apoptosis were measured by Annexin V/PI staining. As shown in Fig. 3, the apoptosis rate was increased upon EGLP treatment in HCT-116 cells. Additionally, the total apoptotic cell populations were up to 26.07% when compared to the control group (P < 0.01). These results indicated that the suppression of HCT-116 cell growth by EGLP is related to apoptosis.
Fig. 3.
EGLP induces the apoptosis of HCT-116 cells. (A–C) HCT-116 cells were treated with EGLP (0, 150, and 300 µg/ml) for 24 h, and the apoptosis of HCT-116 cells was measured by flow cytometry. (D) The apoptosis cells were quantified. The data are reported as mean ± SD (three independent experiments). **P < 0.01 (vs. the control group). EGLP: enzymatically hydrolyzed Ganoderma lucidum polysaccharide; HCT-116: xxx.
EGLP Regulated the Expression of Cell Apoptosis-Related Proteins in HCT-116 Cells
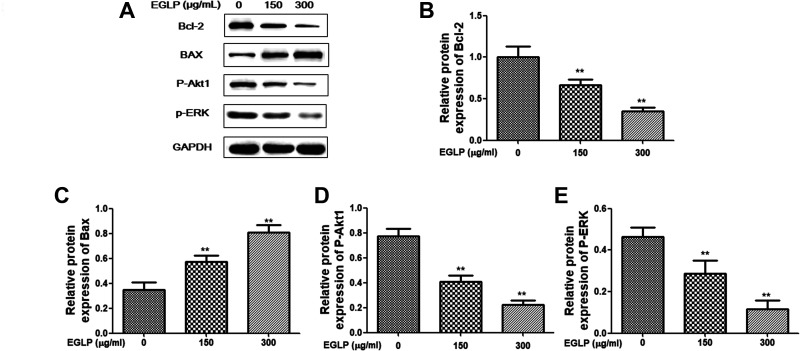
In the present study, western blotting was performed to investigate the underlying mechanism by which EGLP induced cell apoptosis. As shown in Fig. 4, our findings indicated that the protein expressions of Bcl-2, P-ERK, and P-Akt1, which inhibit the cell apoptosis, were decreased in an EGLP dose-dependent manner. Additionally, the cell apoptosis progressive proteins (Bax) were increased in EGLP dose-dependent manner.
Fig. 4.
Effect of EGLP in the apoptotic pathway. HCT-116 cells were treated with EGLP (150 and 300 µg/ml) for 24 h. (A) Western immunoblots of prepared protein and densitometry analysis of protein expression: (B) Bcl-2, (C) Bax, (D) P-Akt1, (E) P-ERK. The results are reported as mean ± SD (three independent experiments). **P < 0.01 (vs. the control group). Bax: xxx; Bcl-2: xxx; EGLP: enzymatically hydrolyzed Ganoderma lucidum polysaccharide; HCT-116: xxx; P-Akt1: xxx; P-ERK: xxx.
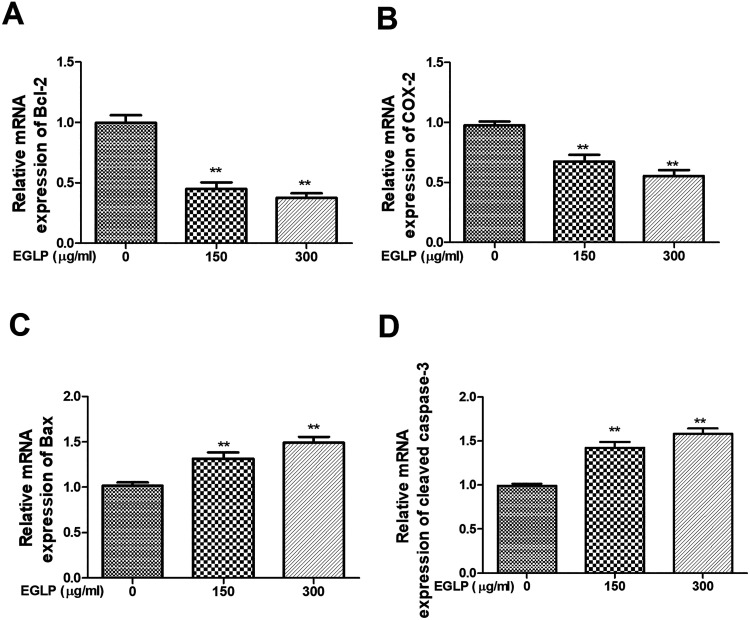
EGLP Regulated the Expression of Cell Apoptosis-Related mRNA in HCT-116 Cells
As shown in Fig. 5, our findings indicated that the mRNA expressions of Bcl-2 and COX-2, which inhibit the cell apoptosis, were decreased in an EGLP dose-dependent manner. Additionally, the mRNA expressions of Bax and cleaved caspase-3, which progress the cell apoptosis, were increased in an EGLP dose-dependent manner.
Fig. 5.
The mRNA expression levels of Bcl-2 (A), COX-2 (B), Bax (C), and cleaved caspase-3 (D) were measured by qRT-PCR assay. The results are reported as mean ± SD (three independent experiments). **P < 0.01 (vs. the control group). Bax: xxx; Bcl-2: xxx; COX-2: xxx; EGLP: enzymatically hydrolyzed Ganoderma lucidum polysaccharide; mRNA: xxx; qRT-PCR: quantitative real-time polymerase chain reaction.
Discussion
Colorectal cancer is one of the most common adenocarcinomas and the third leading cause of tumor-associated incidence and mortality in the world20. At present, the principal treatment of colorectal cancer usually includes chemotherapy and surgical resection. However, organ toxicity and limited effectiveness remain obstacles to the use of those therapies. Therefore, developing a novel tumor inhibitor with low toxicity and high efficiency has become the focus of the present research. Previous reports have indicated that polysaccharides exert effective antitumor effect and broad spectrum21. However, detailed mechanisms and related regulatory aspects remain unclear in colorectal cancer treatment. In our study, the findings indicated that EGLP suppresses the viability of HCT-116 cells in time- and concentration-dependent manner. Besides, EGLP also induces HCT-116 cells apoptosis.
Apoptosis is a general phenomenon in cytotoxicity evoked by antitumor drugs and it is an important mechanism involved in cell death, growth, invasion, and differentiation. Additionally, cell apoptosis is concerned with the malignant tumor progression22. Bcl-2 and Bax are commonly considered as important mediators of cell apoptosis and belong to Bcl-2 families23. A previous report has indicated that the mechanism of Rhizopus nigricans polysaccharide in the promotion of apoptosis in colorectal cancer might be associated with the regulation of the Bax/Bcl-2 signaling pathway24. Additionally, Bax activation and Bcl-2 suppression are considered as a promising therapy for tumor treatment25,26. The previous study has indicated that the improvement in triggering of the mitochondrial pathway by cyclophosphamide might be associated with the effects of GLP on the regulation of Bcl-2 family proteins27, which reminded us to speculate that EGLP may promote HCT-116 cells apoptosis via the regulation of Bax/Bcl-2 protein expression. Lots of reports have indicated that both mitogen-activated protein kinase/extracellular regulated protein kinases (MAPK/ERK) and phosphatidylinositol -3- hydroxykinase (PI3K/Akt) are representative signaling pathways and have been previously indicated to be activated in various types of tumors or cells, including colorectal cancer28,29. Besides, the overexpression of the PI3K/Akt signaling pathway facilitates the depolymerization of Bcl-xl and Bcl-2, resulting in the inactivation of Caspase-9 and Caspase-3, and finally leads to the growth and proliferation of cancer cells29. An accumulation of reports indicated that the activation of COX-2 in various types of tumors is related to the progression of the malignant tumor. Additionally, the COX-2 inhibitor could inhibit the growth of cancer cells and improve the antiangiogenic tumor therapy22. In our research, we observed that EGLP evoked apoptosis in HCT-116 cells. More importantly, the expressions of COX-2, Bcl-2, P-ERK, and p-Akt1 were decreased, and the expressions of Bax and cleaved caspase-3 were increased after EGLP treatment. Previous reports indicated that GLP promotes the apoptosis of Human premortem leukemia cells (HL)-60 cells via regulating the expression of Bax, Bcl-2, and cleaved caspase-3. And enzymatically hydrolyzed G. lucidum crude polysaccharide possesses prior anticervical cancer activity than the crude polysaccharide30,31. The previous findings were in accordance with our results. Our results implied that EGLP might promote colon cancer cells apoptosis via the regulation of p-Akt1/P-ERK and Bax/Bcl-2 protein expression.
Conclusions
In conclusion, the present study showed for the first time that EGLP was capable of inhibiting the growth of HCT-116 cells by promoting apoptosis via the Akt/ERK signaling pathway. Our results provided the scientific evidence for further development of EGLP as a novel antitumor agent for colon cancer treatment.
Footnotes
Ethical Approval: The study was approved by the Institutional Ethics Committee of Cancer Hospital of China Medical University, Shenyang, Liaoning, China.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge the financial support for this work by the National Natural Science Fund from the National Natural Science Foundation of China (Grant No. 81672427).
ORCID iD: Rui Zhang  https://orcid.org/0000-0001-7487-0891
https://orcid.org/0000-0001-7487-0891
References
- 1. Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. [DOI] [PubMed] [Google Scholar]
- 2. Kim YJ, Kang KS, Choi KC, Ko H. Cardamonin induces autophagy and an antiproliferative effect through jnk activation in human colorectal carcinoma HCT116 cells. Bioorg Med Chem Lett. 2015;25(12):2559–2564. [DOI] [PubMed] [Google Scholar]
- 3. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stevens RG, Swede H, Rosenberg DW. Epidemiology of colonic aberrant crypt foci: review and analysis of existing studies. Cancer lett. 2007;252(2):171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gill C, Rowland I. Diet and cancer: assessing the risk. Br J Nutr. 2002;88(Suppl):S73–S87. [DOI] [PubMed] [Google Scholar]
- 6. Fortin O, Aguilar Uscanga BR, Vu KD, Salmieri S, Lacroix M. Effect of saccharomyces boulardii cell wall extracts on colon cancer prevention in male F344 rats treated with 1,2-dimethylhydrazine. Nutr Cancer. 2018;70(4):632–642. [DOI] [PubMed] [Google Scholar]
- 7. Yu Y, Shen M, Song Q, Xie J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr Polym. 2018;183:91–101. [DOI] [PubMed] [Google Scholar]
- 8. Luo J, Zhang C, Liu R, Gao L, Ou S, Liu L, Peng X. Ganoderma lucidum polysaccharide alleviating colorectal cancer by alteration of special gut bacteria and regulation of gene expression of colonic epithelial cells. J Funct Foods. 2018;47:127–135. [Google Scholar]
- 9. Jiang Y, Chang Y, Liu Y, Zhang M, Luo H, Hao C, Zeng P, Sun Y, Wang H, Zhang L. Overview of Ganoderma sinense polysaccharide–an adjunctive drug used during concurrent Chemo/Radiation therapy for cancer treatment in china. Biomed Pharmacother. 2017;96:865–870. [DOI] [PubMed] [Google Scholar]
- 10. Liu ZJ, Wang YL, Li QL, Yang L. Improved antimelanogenesis and antioxidant effects of polysaccharide from cuscuta chinensis lam seeds after enzymatic hydrolysis. Braz J Med Biol Res. 2018;51(7):e7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ji J, Wang LC, Wu H, Luan HM. Bio-function summary of marine oligosaccharides. Int J Biol. 2011;3(1):74–86. [Google Scholar]
- 12. Shao P, Chen X, Sun P. Improvement of antioxidant and moisture-preserving activities of sargassum horneri polysaccharide enzymatic hydrolyzates. Int J Biol Macromol. 2015;74:420–427. [DOI] [PubMed] [Google Scholar]
- 13. Li Q, Zhou S, Jing J, Yang T, Duan S, Wang Z, Mei Q, Liu L. Oligosaccharide from apple induces apoptosis and cell cycle arrest in ht29 human colon cancer cells. Int J Biol Macromol. 2013;57:245–254. [DOI] [PubMed] [Google Scholar]
- 14. Hou Y, Wang J, Jin W, Zhang H, Zhang Q. Degradation of laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr Polym. 2012;87(1):153–159. [DOI] [PubMed] [Google Scholar]
- 15. Fleita D, El-Sayed M, Rifaat D. Evaluation of the antioxidant activity of enzymatically-hydrolyzed sulfated polysaccharides extracted from red algae; pterocladia capillacea. LWT-Food Sci Technol. 2015;63(2):1236–1244. [Google Scholar]
- 16. Pan K, Jiang Q, Liu G, Miao X, Zhong D. Optimization extraction of Ganoderma lucidum polysaccharides and its immunity and antioxidant activities. Int J Biol Macromol. 2013;55:301–306. [DOI] [PubMed] [Google Scholar]
- 17. Zhang T, Tian Y, Jiang B, Miao M, Mu W. Purification, preliminary structural characterization and in vitro antioxidant activity of polysaccharides from acanthus ilicifolius. LWT-Food Sci Technol. 2014;56(1):9–14. [Google Scholar]
- 18. Yousef BA, Hassan HM, Zhang L-Y, Jiang Z-Z. Pristimerin exhibits in vitro and in vivo anticancer activities through inhibition of nuclear factor-кb signaling pathway in colorectal cancer cells. Phytomedicine. 2018;40:140–147. [DOI] [PubMed] [Google Scholar]
- 19. Zou P, Yuan S, Yang X, Zhai X, Wang J. Chitosan oligosaccharides with degree of polymerization 2–6 induces apoptosis in human colon carcinoma HCT116 cells. Chem Biol Interact. 2018;279:129–135. [DOI] [PubMed] [Google Scholar]
- 20. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 21. Sajadimajd S, Momtaz S, Haratipour P, El-Senduny FF, Panah AI, Navabi J, Soheilikhah Z, Farzaei MH, Rahimi R. Molecular mechanisms underlying cancer preventive and therapeutic potential of algal polysaccharides. Curr Pharm Des. 2019;25(11):1210–1235. [DOI] [PubMed] [Google Scholar]
- 22. Zhou H, Yang J, Zhang C, Zhang Y, Wang R, Li X, Zhang S. Safflower polysaccharide inhibits the development of tongue squamous cell carcinoma. World J Surg Oncol. 2018;16(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281(5):E924–E930. [DOI] [PubMed] [Google Scholar]
- 24. Song G, Lu Y, Yu Z, Xu L, Liu J, Chen K, Zhang P. The inhibitory effect of polysaccharide from rhizopus nigricans on colitis-associated colorectal cancer. Biomed Pharmacother. 2019;112:108593. [DOI] [PubMed] [Google Scholar]
- 25. Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, et al. Abt-199, a potent and selective bcl-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. [DOI] [PubMed] [Google Scholar]
- 26. Xin M, Li R, Xie M, Park D, Owonikoko TK, Sica GL, Corsino PE, Zhou J, Ding C, White MA, Magis AT, et al. Small-molecule bax agonists for cancer therapy. Nat Commun. 2014;5:4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li W, Nie S, Chen Y, Wang Y, Li C, Xie M. Enhancement of cyclophosphamide-induced antitumor effect by a novel polysaccharide from Ganoderma atrum in sarcoma 180-bearing mice. J Agric Food Chem. 2011;59(8):3707–3716. [DOI] [PubMed] [Google Scholar]
- 28. Ma R, Wang L, Yuan F, Wang S, Liu Y, Fan T, Wang F. Fabp7 promotes cell proliferation and survival in colon cancer through MEK/ERK signaling pathway. Biomed Pharmacother. 2018;108:119–129. [DOI] [PubMed] [Google Scholar]
- 29. Zhang R, Yu Q, Lu W, Shen J, Zhou D, Wang Y, Gao S, Wang Z. Grape seed procyanidin b2 promotes the autophagy and apoptosis in colorectal cancer cells via regulating pi3k/akt signaling pathway. Onco Targets Ther. 2019;12:4109–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang G, Yang L, Zhuang Y, Qian X, Shen Y. Ganoderma lucidum polysaccharide exerts anti-tumor activity via mapk pathways in hl-60 acute leukemia cells. J Recept Signal Transduct Res. 2016;36(1):6–13. [DOI] [PubMed] [Google Scholar]
- 31. Kong M, Yao Y, Zhang H. Antitumor activity of enzymatically hydrolyzed Ganoderma lucidum polysaccharide on U14 cervical carcinoma-bearing mice. Int J Immunopathol Pharmacol. 2019;33:2058738419869489. [DOI] [PMC free article] [PubMed] [Google Scholar]