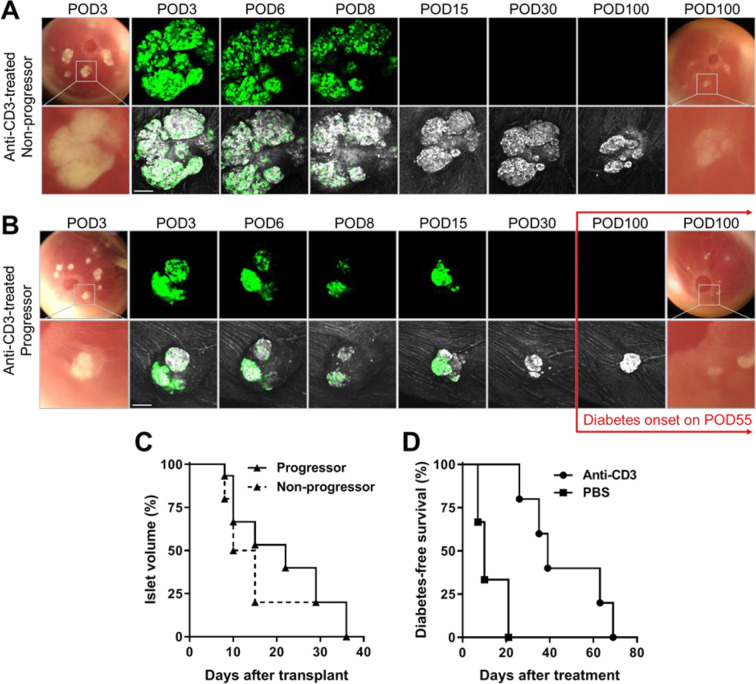
Figure 2.
Anti-CD3 antibody treatment delayed autoimmune T1D onset but not the destruction of GFP-expressing beta cells. (A, B) Representative images and confocal micrographs of NODMIPGFP islets transplanted in the ACE of late prediabetic female NOD mice that either (A) remained diabetes-free (nonprogressor) or (B) progressed to diabetes/hyperglycemia (progressor). Islets were clearly visible initially on top of the iris as dense white masses (bottom rows) and corresponding GFP signal (top rows) and became less dense and visible as they were attacked and destroyed. Both progressors and nonprogressors were treated with anti-CD3 mAb and treatment was started on (A) POD10 and (B) POD8 and was maintained for five consecutive days19,22,26 (see Methods for details). Confocal micrographs were acquired as z-stacks and are shown as maximum intensity projections. Scale bars = 100 µm. (C) Kaplan–Meier curves showing the destruction kinetics of NODMIPGFP NOD islets in the progressors (n = 15 islets in three mice) and nonprogressors (n = 15 islets in two mice). (D) Kaplan–Meier curves showing diabetes-free survival of progressors that received ACE-transplanted NODMIPGFP islets (GFP+) and were treated with PBS (n = 3 mice) or anti-CD3 mAb (n = 5 mice). ACE: anterior chamber of the eye; GFP: green fluorescent protein; mAb: monoclonal antibody; NOD: nonobese diabetic; PBS: phosphate-buffered saline; POD: postoperative day.