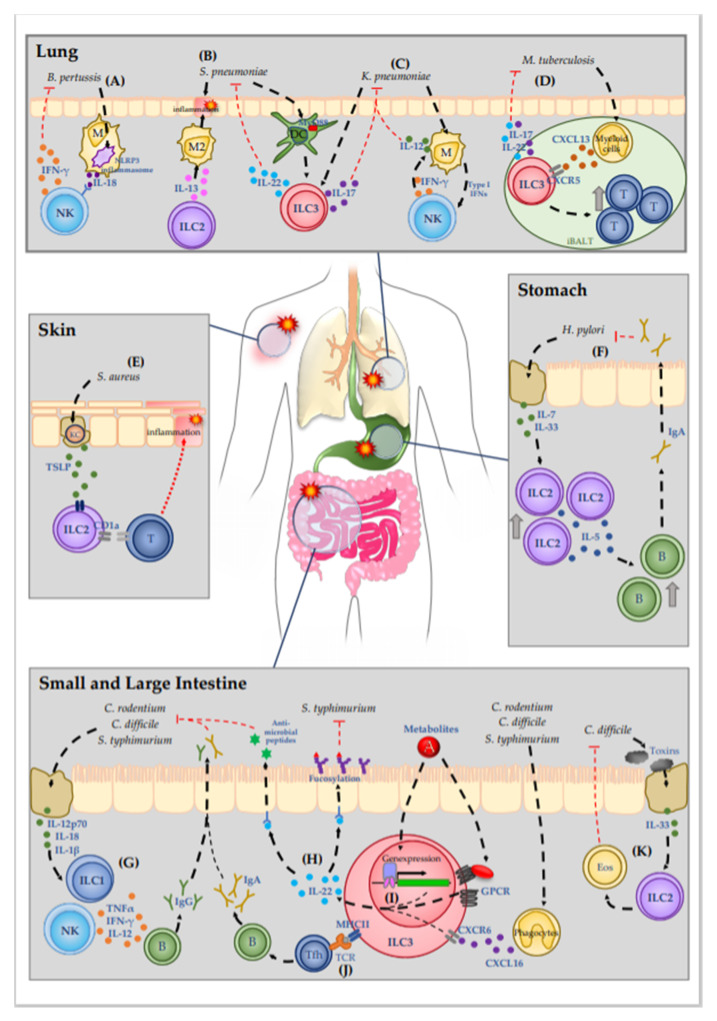
Figure 1.
Molecular mechanisms of ILC-mediated immune defense against bacterial infections.Mucosal tissues are sites that are especially prone to bacterial infection due to the high prevalence of pathogenic bacteria. (A) B. pertussis causes the severe respiratory infection pertussis. Detection of B. pertussis by macrophages initiates IL-18 release via NLPR3 inflammasome activation, which promotes IFN-γ release from NK cells. (B,C) In the lung pneumonia can be caused by the pathogens S. pneumoniae and K. pneumoniae. (B) ILC-2-drived IL-13 mediates early polarization of alveolar macrophages into the M2 phenotype. The quiescent immune environment delays the immune response against S. pneumoniae. S. pneumoniae is further detected by DC, which then activate ILC3 in a MyD88-dendent manner, resulting in the production of IL-22. (C) ILC3 are also activated during K. pneumoniae infection, increasing IL-17 expression. A mutual interaction of macrophages and NK cells results in IL-12 release from macrophages that plays a critical role in K. pneumoniae containment. (D) M. tuberculosis infection induces CXCL13 secretion by myeloid cells in the lymphoid tissue. CXCL13 activates ILC3 via CXCR5, inducing the secretion of IL-22 and IL-17. Moreover, activated ILC3 promotes lymphocyte recruitment to the iBALT site, thus inhibiting infection progression. (E) Patients with atopic dermatitis are susceptible for S. aureus infection. Lesional keratinocytes release TSLP, which increases CD1a expression on ILC2, which activates CD1a-reactive T cells and leads to the recruitment of further immune cells. (F) H. pylori infection in the stomach leads to epithelial cell damage, thus inducing release of IL-7 and IL-33. Enhanced cytokine secretion boosts ILC2 recruitment and activation and simultaneously enhances the expression of IL-5. IL-5 promotes the recruitment of B cells and concomitant production of IgA. In the stomach lumen, IgA neutralizes the pathogen, thus decreasing bacterial burden in the tissue. (G–K) In the intestinal tract, pathogens like C. rodentium, C. difficile, and S. typhimurium cause inflammation with various symptoms. (G) Damage to epithelial cells induces release of interleukins such as IL-12p70, IL-18 and IL-1β, thus activating ILC1. ILC1-derived TNF-α, IFN-γ, and IL-12 promote the generation of pathogen specific IgG. (H–J) The most well-studied ILC subgroup in terms of bacterial infections is ILC3. (H) Activated ILC3 mediate bacterial infection protection mainly via the secretion of IL-22, which initiates the secretion of antimicrobial peptides from epithelial cells as well as epithelial cell fucosylation, thus impeding bacterial colonization. (I) IL-22 expression by ILC3 can be induced by various stimuli: By metabolites, which derive from the gut lumen and activate GPCR receptors or directly modulate gene expression of IL-22 promoting genes, or by the activation of CXCR6 via phagocyte-derived CXCL16. (J) Moreover, MHC class II expression on ILC3 allows the direct presentation of pathogen-derived peptide antigens to T cells. promoting the generation of pathogen-specific IgA. (K) C. difficile-secreted toxins induce epithelial cell damage, resulting in IL-33 release. IL-33 activates ILC2, which promote the recruitment of eosinophils, thereby supporting the clearance of the pathogen and the healing of the epithelial layer. Abbreviations: DC—dendritic cells; M2—type 2 macrophages; T—T cells; KC—Keratinocytes; B—B cells; Eos—Eosinophils; GPCR—G-protein-coupled receptors.