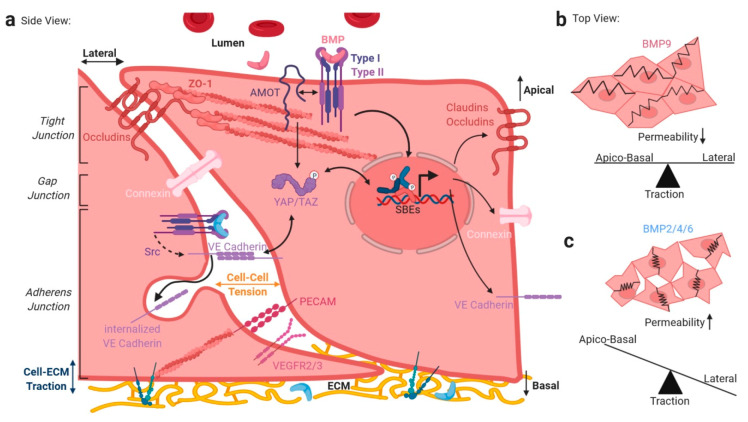
Figure 4.
Crosstalk of mechanical and TGFβ/BMP signaling at endothelial cell junctions. EC junctions are crucial for cell–to-cell communications and barrier function (a). Apical TJs, composed mainly of claudins and occludins connect to the cytoskeleton via zona occludens (ZO) proteins, regulating trans-endothelial macromolecule transport by narrowing the inter-endothelial junction size. AJ-related protein AMOT regulates the apical BMP signaling by interaction with BMP receptors. Gap junctions allow communication between neighboring ECs via pore-forming proteins like connexins. Basal AJs constitute connection points of cell–ECM and cell–cell interactions. The main components are the trans-interacting proteins VE-Cadherin and PECAM-1, together with VEGFR2/3, which form a mechanosensory complex, necessary for FSS sensation. VE-Cadherin internalization and thus AJ resolution is regulated by BMP signaling via Src. Additionally, VE-Cadherin associated β-Catenin complex interacts with YAP/TAZ in the cytoplasm, and YAP/TAZ interacts with SMADs proposed to influence SMAD stability, shuttling, and transcriptional competence. SMAD gene transcription regulates the expression of several junctional proteins like claudins, occludins, connexins, or VE-Cadherin. Here, distinct BMP ligands exhibit different potentials of junction regulation and thus cell–cell and cell–ECM traction (a). As proposed on the right, homeostatic BMP9 might decrease EC permeability by keeping a balance of apical–basal and lateral traction (b). In contrast, BMP2/4/6 might increase EC permeability by shifting towards apico-basal traction (c). Abbreviations: SBE-—MAD binding elements, TJs—Tight junctions, AJs—Adherens junctions.