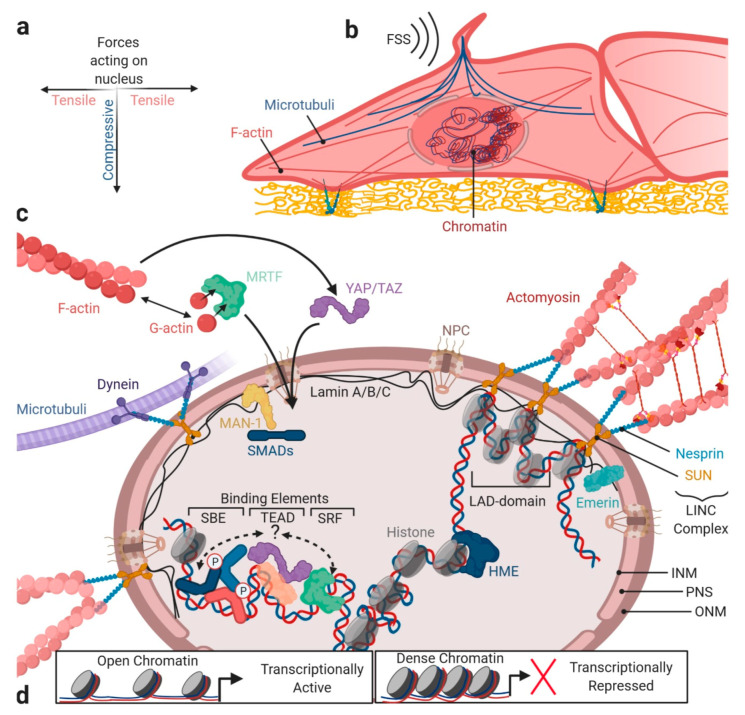
Figure 6.
TGFβ/BMP signaling crosstalk and nuclear mechanics. The nucleus is connected to the PM, including the primary cilium, junctions, and cell–ECM contacts via the F-actin fibers and microtubules. Thereby, the nucleus is indirectly exposed to external mechanical forces acting in a tensile (via F-actin) and compressive (via microtubules) fashion (a), e.g., induced by FSS (b). In particular, the filaments are connected to the inner and outer nuclear membrane (INM/ONM) via LINC complexes, composed of the nesprin and SUN proteins (c). For microtubule, connection to LINC complexes is mediated via dynein. By transmission of external forces, opening of NPCs might be regulated, allowing a more efficient influx of TFs, like SMADs or F/G-actin-ratio-sensing MRTF, or co-factors like YAP/TAZ. TFs can regulate the transcription of target genes in regions of “open” heterochromatin. SMADs can direct HMEs to target gene sequences, thereby allowing epigenetic modification and thus the activation or repression of genes (d). Chromatin compartments of inactive euchromatin are merely found to be proximal to the INM in lamin-associated LADs. Additional to lamins, integral proteins like MAN-1 sit in the NM. MAN-1 confers a further layer of BMP signaling regulation, by binding SMADs and competing with the co-factor binding. Abbreviations: NPC—nuclear pore complex, ONM—outer nuclear membrane, PNS—perinuclear space, INM—inner nuclear membrane, LAD—lamina-associated domain, HME—histone modifying enzyme, and SBE—SMAD binding element.