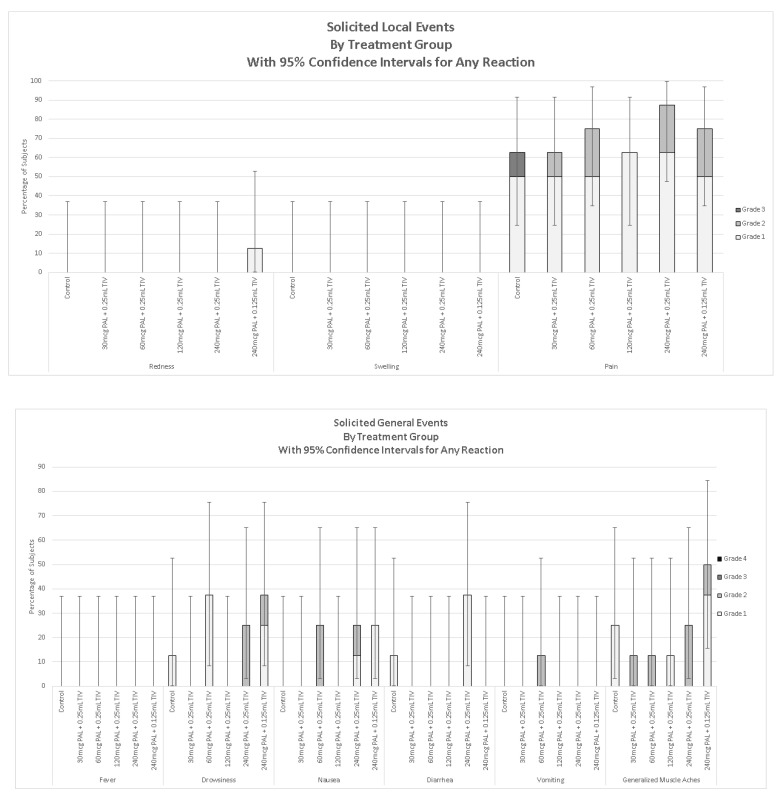
Figure 2.
Solicited local and systemic adverse events in the seven days after vaccination. Legend. Control–Trivalent inactivated influenza vaccines (TIV) 0.5 mL; 30 µg PAL adjuvant and 0.25 mL TIV; 60 µg PAL adjuvant and 0.25 mL TIV; 120 µg PAL adjuvant and 0.25 mL TIV; 240 µg PAL adjuvant and 0.25 mL TIV, 240 µg of PAL adjuvant and 0.125 mL TIV. Redness or swelling grade 1—>20–≤50 mm diameter; Grade 2—>50–≤100 mm diameter, Grade 3—>100 mm; Pain at injection site Grade 1—mild, neither interfering nor preventing normal every day activities, Grade 2—moderate, painful when limb is moved and interferes with normal every day activities, Grade 3—severe, significant pain at rest. Prevents normal every day activities. Error bars show 95% confidence intervals.