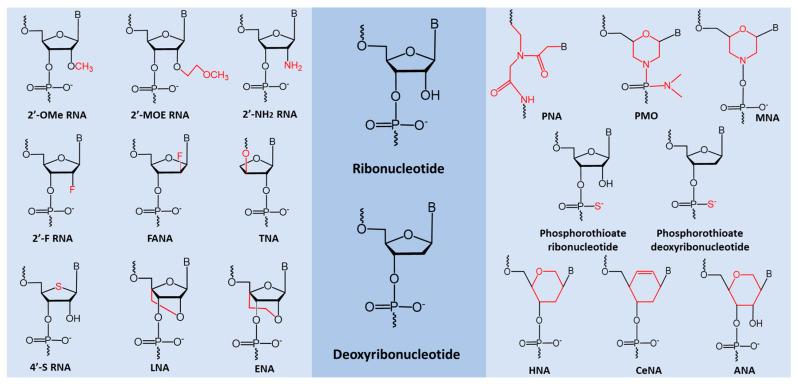
Figure 3.
Examples of nucleotide analogues used in oligonucleotide synthesis. 2′-OMe, 2′-O-methyl [250,251]; 2′-MOE, 2′-O-methoxyethyl [252]; 2′-NH2, 2′-amino [253]; 2′-F, 2′-fluoro [254]; 2′-FANA, 2′-fluroarabino nucleic acid [255]; TNA, threose nucleic acid [256]; 4′-S, 4′-thio [257]; LNA, locked nucleic acid [258,259,260]; ENA, 2′-O, 4′-C-ethylene-bridged nucleic acid [261]; PNA, peptide nucleic acid [262]; PMO, phosphorodiamidate morpholino oligomer [263]; MNA, morpholino nucleic acid [264]; Phosphorothioate, PS [265]; HNA, 1,5-anhydro hexitol nucleic acid [266]; CeNA, cyclohexenyl nucleic acid [266]; ANA, altritol nucleic acid [266].