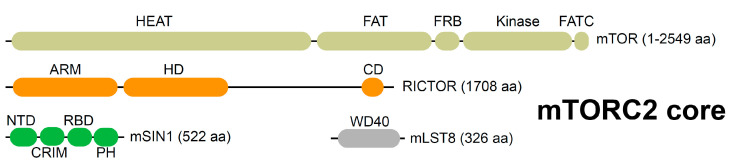
Figure 1.
Composition of mTORC2 core. mTORC2 is composed of four core subunits (mTOR, RICTOR, mSIN1, and mLST8). mTOR is composed of several domains, including the HEAT (huntingtin, elongation factor 3, a subunit of protein phosphatase 2A, TOR1; a tandemly repeated motif with helical structure), FAT (FRAP, ATM, TRRAP; α helices arranged as repeats), FRB (FKBP12–rapamycin binding), kinase, and FATC (FRAP, ATM, TRRAP, C-terminal; an α-helix and a disulfide-bonded loop) domains. RICTOR has armadillo (ARM; two curved layers of α-helix) repeats, a HEAT-like domain (HD), and a C-terminal domain (CD), and all three regions likely interact with mSIN1. A flexible region between HD and CD contains most of the identified RICTOR phosphorylation sites. mSIN1 is composed of NTD (N-terminal domain), CRIM (conserved region in the middle; substrate recruitment), RBD (Ras binding domain), and PH (pleckstrin homology; has membrane targeting capacity) domains. NTD and CRIM regions of mSIN1 have direct contact with RICTOR and mLST8. mLST8 is composed mainly of WD40 repeats and interacts with the kinase domain of mTOR.