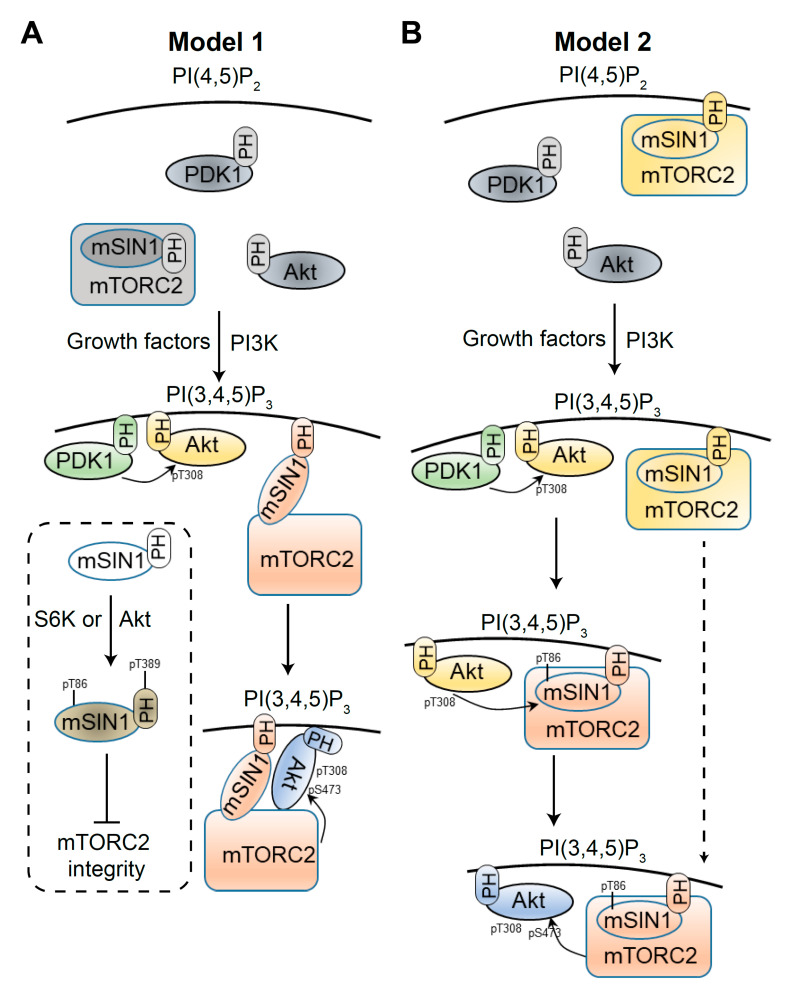
Figure 2.
Growth factors induce Akt Ser473 phosphorylation via mTORC2. In both models, in the absence of growth factors, PDK1 and Akt are localized at the cytoplasm. Upon growth factor stimulation, PI(4,5)P2 is converted to PI(3,4,5)P3 via PI3K at the plasma membrane, and PDK1 and Akt are recruited to the plasma membrane via their pleckstrin homology (PH) domains, leading to phosphorylation of Akt Thr308 by PDK1. In model 1 (A), PI(3,4,5)P3 recruits mTORC2 to the plasma membrane and directly activates mTORC2 toward Akt Ser473 via the release of an inhibitory conformation. In the box, S6K or Akt leads to dual phosphorylation of mSIN1 at Thr86 and Thr389 to inhibit mTORC2. In model 2 (B), mTORC2 permanently resides at the plasma membrane. Akt-pThr308 phosphorylates mSIN1 Thr86 within mTORC2, which in turn boosts mTORC2 activity. mTORC2 then phosphorylates Akt Ser473, leading to full activation of Akt. The contribution of mTORC2 containing Thr86-nonphosphorylated mSIN1 to Akt Ser473 phosphorylation is not very clear.