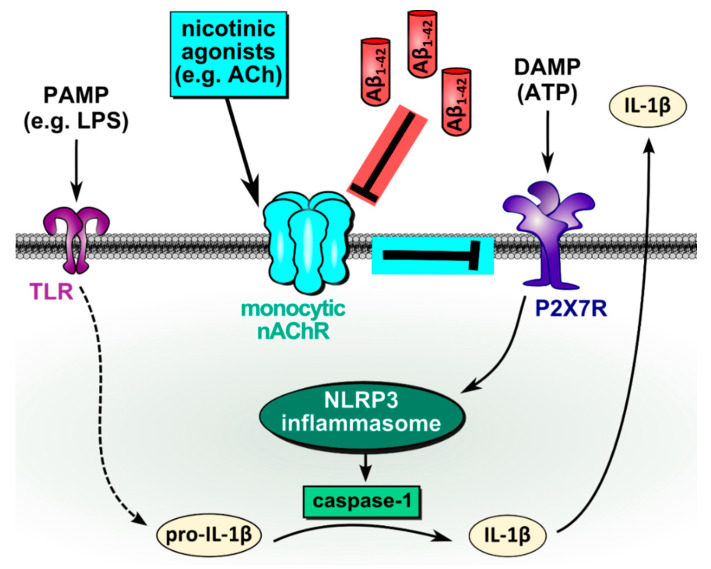
Figure 6.
Schematic summary of the proposed mechanism. In LPS-primed monocytic cells, extracellular ATP, originating from dead or injured cells, binds to the ATP-sensitive P2X7R, induces NLRP3 inflammasome assembly, activation of caspase-1, cleavage of pro-IL-1β and release of bioactive IL-1β. This damage-associated release of IL-1β is inhibited by the activation of monocytic nAChRs containing subunits α9, α7, and/or α10. As shown previously, activation of nAChRs inhibits the ion channel function of P2X7R and, thus, ATP-induced IL-1β release [6,7]. Our data suggest that amyloid beta (Aβ1-42) antagonizes this inhibitory effect and enables monocytic release of IL-1β in the presence of nicotinic agonists. ACh, acetylcholine; ATP, adenosine triphosphate; DAMP, danger-associated molecular pattern; IL-1β, interleukin-1β; LPS, lipopolysaccharide; nAChR, nicotinic acetylcholine receptor; NLRP3, NACHT, LRR, and PYD domains-containing protein 3; P2X7R, P2X7 receptor; PAMP, pathogen-associated molecular pattern; TLR, Toll-like receptors.