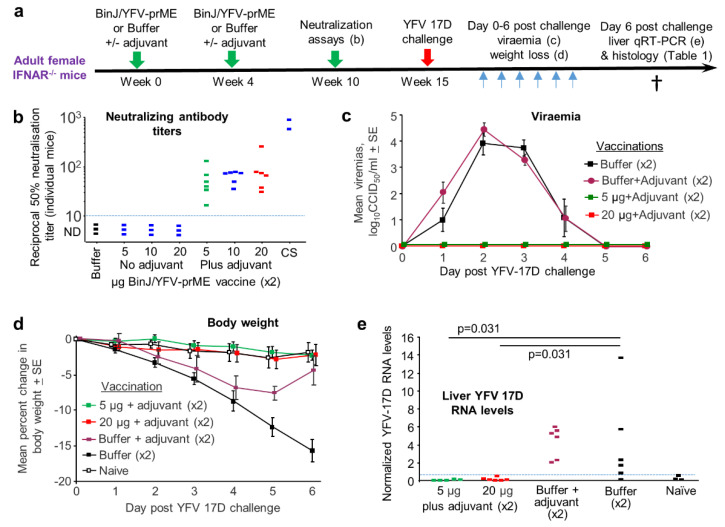
Figure 3.
Evaluation of the BinJ/YFV-prME vaccine in female IFNAR-/- mice. (a) Time course of experiment. (b) Neutralizing antibody responses for No adjuvant and Plus adjuvant groups with the indicated dose of vaccine (µg) given twice (×2). CS–pooled convalescent serum taken from IFNAR-/- mice infected 6–10 weeks previously with YFV 17D. Limit of detection was a dilution of 1 in 10 (dotted line). (c) Three groups from b (indicated by black, green and red squares) were challenged with YFV 17D and viraemia post challenge shown (limit of detection for each mouse is 2 log10CCID50/mL). (d) Percent weight loss for the mice in c. A fourth group is included (n = 5) who were bled daily as for the infected mice, but that received no vaccination or challenge. Differences between Buffer and BinJ/YFV-prME vaccinated mice were significant on days 3 through 6 (p < 0.01, t tests). (e) qRT PCR for YFV 17D in livers taken day 6 post challenge in the indicated groups. Horizontal line indicates cut-off for reliable detection, with multiple repeat tests of Naïve livers falling below this line. Statistics by Kolmogorov Smirnov tests.