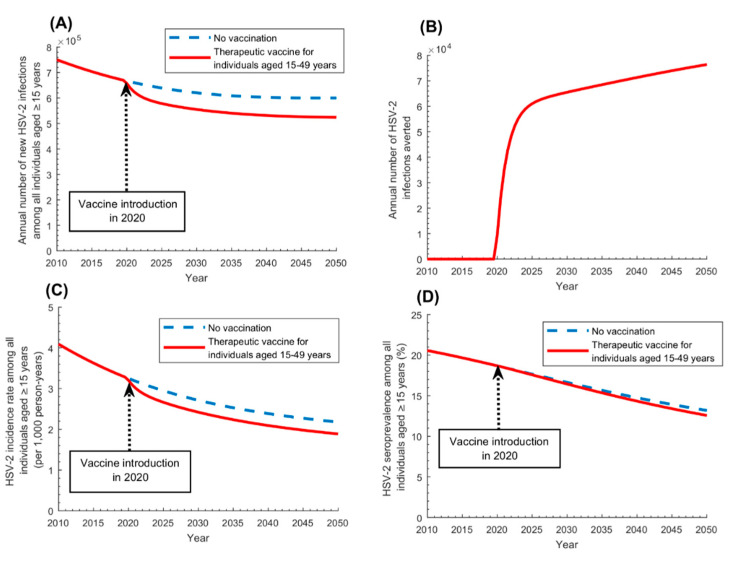
Figure 6.
Impact of therapeutic HSV-2 vaccination administered to infected persons with symptomatic disease aged 15–49 years on HSV-2 infection measures in the population aged ≥15 years. Impact of the therapeutic vaccine on (A) annual number of new HSV-2 infections, (B) annual number of HSV-2 infections averted, (C) HSV-2 incidence rate, and (D) HSV-2 seroprevalence, among those aged ≥15 years. The therapeutic vaccine is introduced in 2020, with its coverage scaled up to 80% by 2030, and maintained at this level thereafter. Duration of vaccine-induced protection is 10 years and VEP is 50%.