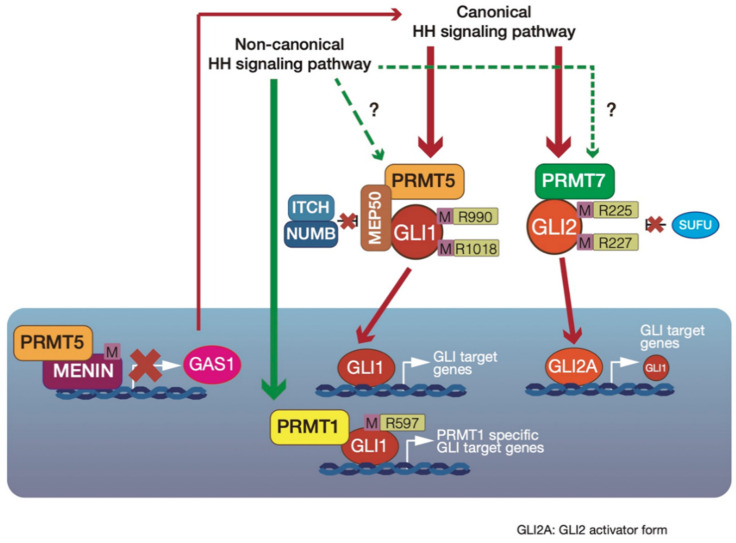
Figure 4.
Protein arginine methyl transferase (PRMT)-mediated modulation of glioma-associated oncogene (GLI) 1 or GLI2 activity. PRMT1 methylates an arginine residue at 597 in GLI1. This PRMT1-mediated GLI1 methylation assists with the upregulation of GLI1 activity. Furthermore, methylated GLI1 enhances the expression of some specific GLI1 target genes (IGFBP6 and BCL2). Cytoplasmic PRMT5 methylates arginine residues at 990 and 1018 in GLI1. This PRMT5-mediated GLI1 methylation inhibits the interactions of GLI1 and ITCH–Numb E3 ligase, and stabilizes the GLI1 protein. In contrast, nucleic PRMT5 interacts with Menin and represses growth arrest-specific protein (GAS) 1, and indirectly downregulates the HH signaling pathway. PRMT7 methylates arginine residues at 225 and 227 in GLI2. This PRMT7-mediated GLI2 methylation inhibits the binding of Suppressor of Fused (SUFU) to GLI2, resulting in GLI2 activation.