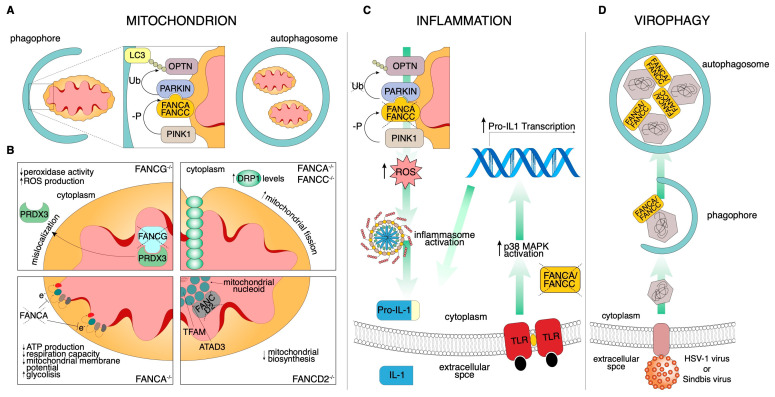
Figure 2.
Schematic of the noncanonical FA pathways. (A) During mitophagy, damaged mitochondria are selectively engulfed by the phagophore. To properly identify the substrate, the E3 ligase PARKIN ubiquitinates the mitophagy receptor, which acts as an adapter for LC3-mediated autophagosome recognition. In this context, FANCC assists PARKIN localization to the mitochondria. (B) FA protein-related mitochondrial functions. (Top left) FANCG-deficient cells display PRDX3 mislocalization. The consequent reduced mitochondrial peroxidase activity increases ROS production. (Top right) FANCA and FANCC downregulated cells show increased DRP1 levels resulting in an increment of mitochondrial fission. (Bottom left) FANCA deficiency impacts negatively on mitochondrial electron transport chain efficiency, reducing ATP production and respiration capacity and altering the mitochondrial membrane potential. This inevitably leads to a shift from aerobic to glycolytic metabolism. (Bottom right) FANCD2 mitochondrial nucleoid complex interaction regulates mitochondrial biosynthesis. (C) FANCA and FANCC knockdown increase the pro-inflammatory secretion of IL-1β in a dual convergent manner. On the left, impaired mitophagy stimulates inflammasome activation through ROS overproduction and, consequently, IL-1β is secreted. On the right, exogenously stimulated Toll-like receptors activate a signal transduction cascade that culminates with Pro-IL-1β transcription. (D) Both FANCA and FANCC are required for Sindbis and HSV-1 nucleocapsid recognition to control viral infection by virophagy.