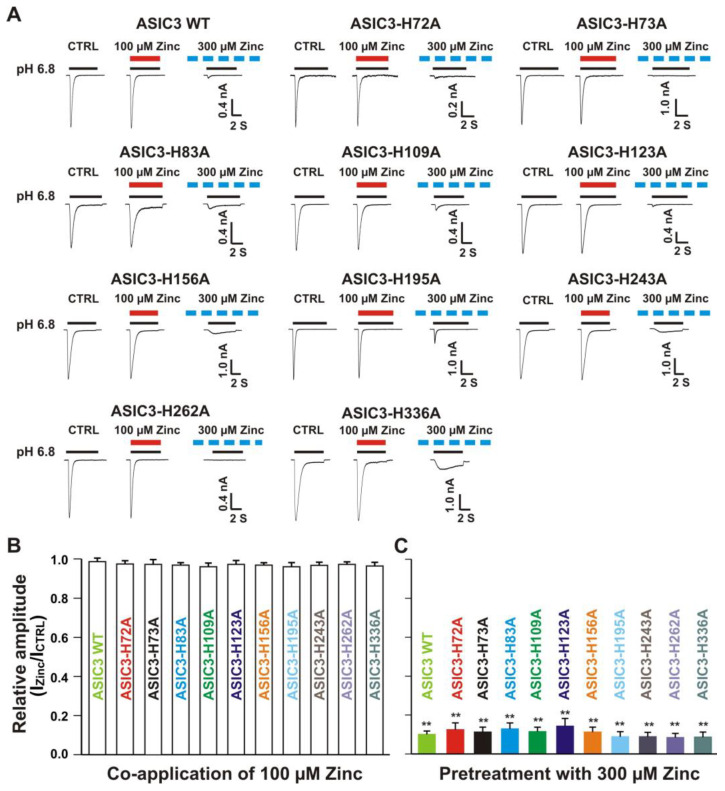
Figure 11.
Zinc effects on currents from ASIC3 WT and ASIC3 mutants of histidine residues in the extracellular domain of ASIC3. (A) Representative traces show that co-application of zinc at a concentration of 100 µM had no effect on peak amplitude of the currents recorded from ASIC3 WT, ASIC3-H72A, ASIC3-H73A, ASIC3-H83A, ASIC3-H109A, ASIC3-H123A, ASIC3-H156A, ASIC3-H195A, ASIC3-H243A, ASIC3-H262A, and ASIC3-H336A; whereas pretreatment with zinc at a concentration of 300 µM showed profound inhibition on currents from ASIC3 WT, ASIC3-H72A, ASIC3-H73A, ASIC3-H83A, ASIC3-H109A, ASIC3-H123A, ASIC3-H156A, ASIC3-H195A, ASIC3-H243A, ASIC3-H262A, and ASIC3-H336A. The solid red and black lines represent 100 µM zinc and pH 6.8 application, respectively (each recoding with 7 s of zinc and pH 6.8 application). The dashed blue line represents pretreatment with 300 µM zinc in the pH 7.4 solution with a duration of 2 min. (B and C) Statistical bar graphs show relative peak amplitudes of the currents by co-application of zinc (B, n = 7 to 10) and pretreatment with zinc on ASIC3 WT and their histidine mutants expressed on CHO cells as mentioned above (C, n = 6 to 10). There was no significant difference on currents by co-application of zinc or pretreatment with zinc (inhibitory effect) among ASIC3 WT and their histidine mutants (p > 0.05, ANOVA). For ASIC3 mutation, histidine residues in the extracellular domain of ASIC3 were replaced with alanine. Whole-cell patch-clap recording was performed and currents of ASIC3 and its mutants were activated by a drop in pH from 7.4 to 6.8. Data are presented as mean ± SEM. CTRL represents control. ICTRL, ASIC current without any treatment; IZinc, ASIC current by zinc treatment. ** p < 0.01 (t-test).