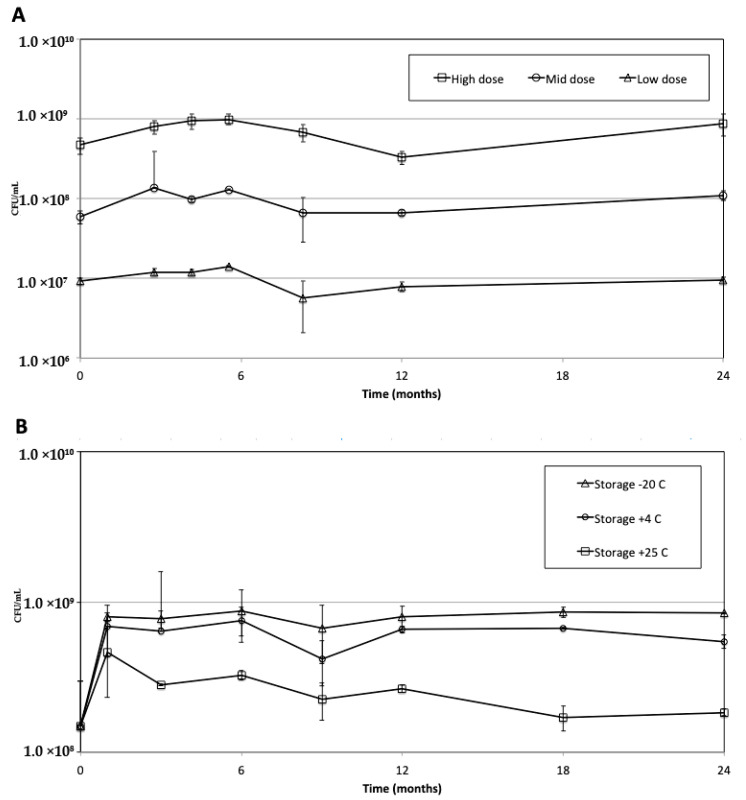
Figure 3.
Microbiological stability of the BPZE1 drug products over time. (A) The liquid BPZE1 drug product at 107 CFU/dose (triangles, low dose), 108 CFU/dose (circles, middle dose) and 109 CFU/dose (squares, high dose) was stored at −70 °C for two years, and CFU counts were conducted at the indicated time points. (B). Microbiological stability of the lyophilized BPZE1 drug product over time. The lyophilized BPZE1 drug product at 109 CFU/dose was stored at −20 °C ± 10 °C (triangles), 5 °C ± 3 °C (circles) and 22.5 °C ± 2.5 °C (squares) for two years, and CFU counts were conducted at the indicated time points. Vertical lines represent standard deviations.