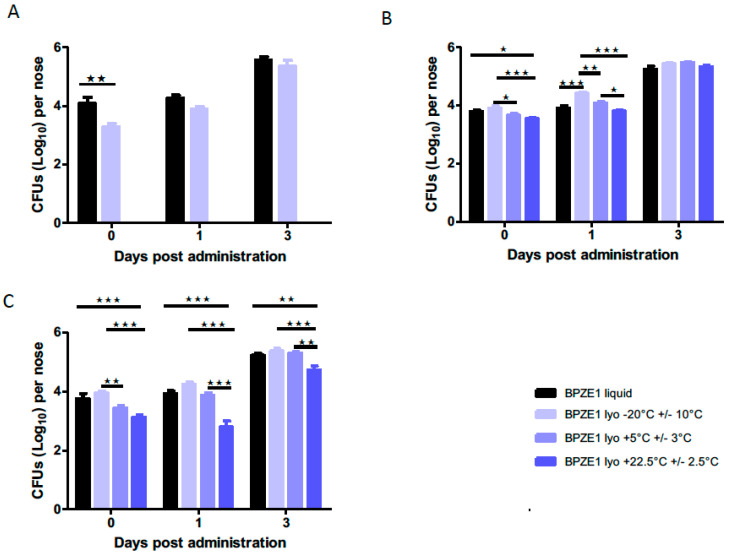
Figure 4.
In-vivo colonization kinetics of the lyophilized BPZE1 drug product compared to the liquid drug product. BALB/c mice (n = 5 per group and per time point) were inoculated intranasally with 105 CFU of the liquid BPZE1 drug product (black bars) or the reconstituted lyophilized BPZE1 drug product (blue bars) and sacrificed 3 h (day 0), 1 or 3 days thereafter to evaluate the CFU numbers in the nasal homogenates. (A) Comparison of the CFU counts of the liquid BPZE1 drug product with those of the lyophilized BPZE1 drug product reconstituted and administered immediately after lyophilization. (B) Comparison of the CFU counts of the liquid BPZE1 drug product with those of the lyophilized BPZE1 drug product reconstituted 6 months after storage at −20 °C ± 10 °C (light blue lines), 5 °C ± 3 °C (middle blue lines) or 22.5 °C ± 2.5 °C (dark blue lines). (C) Comparison of the CFU counts of the liquid BPZE1 drug product with those of the lyophilized BPZE1 drug product reconstituted 24 months after storage at −20 °C ± 10 °C (light blue lines), 5 °C ± 3 °C (middle blue lines) or 22.5 °C ± 2.5 °C (dark blue lines). The results are expressed as means +/– SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.005; ns, not significant.