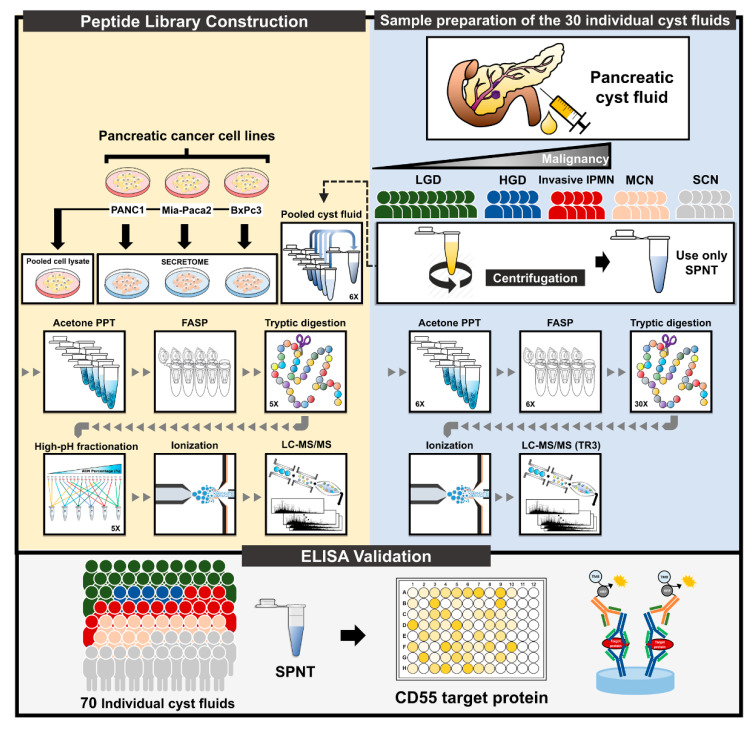
Figure 1.
Experimental workflow. The overall experimental workflow comprises 3 sections: (1) Preparation of 30 individual samples, (2) peptide library construction, and (3) validation by ELISA. The cohort for label-free quantification included 30 pancreatic cyst fluid samples (10 LGD, 5 HGD, 5 invasive IPMN, 5 MCN, and 5 SCN). After mucus removal by sonication, the samples were centrifuged to isolate supernatant. Pooled cyst fluid (comprising equal amounts of 30 individual samples), secreted proteins from PANC1, Mia Paca-2, BxPC3, and pooled cell lysates from the 3 cell lines were compiled to generate a peptide library. All samples were precipitated using cold acetone to extract the protein. After FASP digestion, only the samples that were used to construct the peptide library were subjected to high-pH reverse-phase peptide fractionation. All peptides were analyzed on a Q Exactive mass spectrometer. CD55, one of the potential markers of IPMN dysplasia, was validated by ELISA. PPT, precipitation; FASP, filter-aided sample preparation; LGD, low-grade dysplasia; HGD, high-grade dysplasia; MCN, mucinous cystic neoplasm; SCN, serous cystic neoplasm; SPNT, supernatant.