Figure 3.
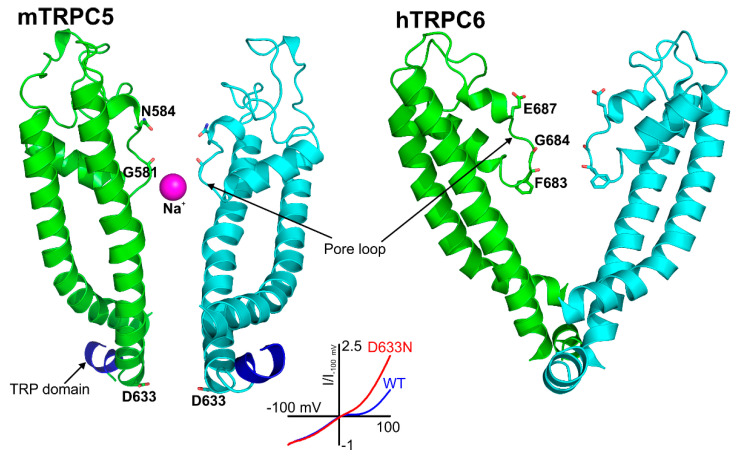
Structural architecture of the pore region of TRPC5 and TRPC6 channels. Only two subunits are shown for clarity (mouse TRPC5-pdb: 5aei and human TRPC6-pdb: 5yx9). The residues involved in controlling TRPC5 and TRPC6 cation selectivity are indicated within the pore loop of the channels. The role of the E687 residue in controlling TRPC6 Ca2+ permeability was identified by the Klaus Groschner group in 2011 [104,105], whereas the importance of the N584 residue for determining the TRPC5 channel’s Ca2+ selectivity was identified in a screen by Chen et al. in 2017 [56]. TRPC5 is inhibited by intracellular Mg2+ in a voltage-dependent manner, with a S6 transmembrane helix residue, D633, being responsible for that signature property of TRPC5 [79]. The inset shows the current–voltage relationships of wild type and the D633N mutant of TRPC5. The D633N mutant exhibits a reduced Mg2+ sensitivity, whereas the D636N mutant has a current–voltage relationship similar to that of the wild type TRPC5 [79]. The solved structure of TRPC5 confirmed that the D633 residue is located within the cation conduction pathway, whereas neighboring D636 residue faces away [103]. The mouse TRPC5 and human TRPC6 atomic coordinates were from PDB ID#: 5AEI and PDB ID#: 5YX9, respectively.