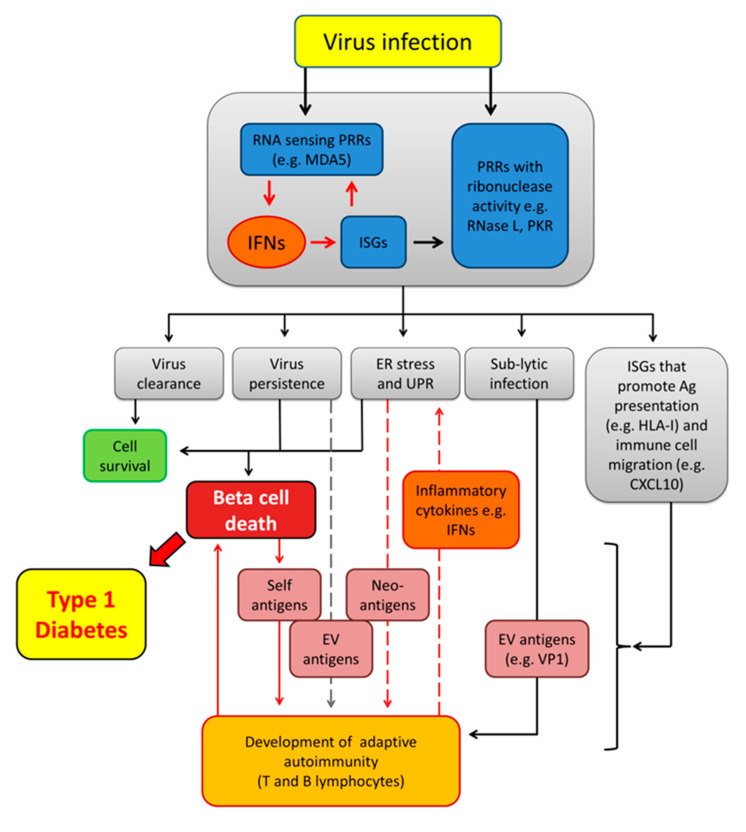
Figure 1.
Virus-induced autoimmune T1D. An initial enterovirus (EV) infection activates pattern recognition receptors (PRRs) that induce interferons (IFNs) and interferon stimulated genes (ISGs) as well as PRRs that directly target and cleave viral RNA, driving a collective intrinsic antiviral response that may lead to virus clearance or virus persistence. The surge in IFNs and ISGs promotes Endoplasmic Reticulum (ER) stress and unfolded protein response (UPR) which, alone or in combination with virus infection, may lead to programmed cell death (apoptosis), exposing virus and self-antigens to the immune system. In addition, IFNs promote antigen presentation and immune cell migration by inducing the expression of human leukocyte antigen class I (HLA-I) molecules and chemokines such as C-X-C motif chemokine 10 (CXCL10). Cells with lytic or persistent infection that do not undergo apoptosis may present viral antigens (e.g., VP1) and self-antigens (including neo-antigens induced by IFNs and UPR) to the immune cells (APCs, CD8+ and CD4+ T cells) leading to development of autoantibodies and an adaptive autoimmune response against antigen producing cells. Islet infiltrating immune cells may also contribute to ER stress and UPR in beta cells by releasing inflammatory cytokines such as IFNα and IFNγ. MDA5, melanoma differentiation-associated protein 5; PKR, protein kinase R; VP1, virus capsid protein 1.