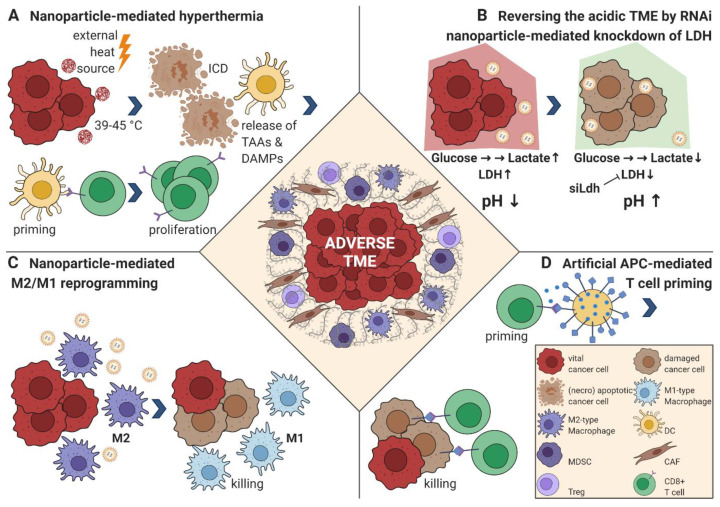
Figure 2.
Strategies to reprogram the suppressive TME. (A) Targeted nanoparticles are administered and home to the TME. Upon external irradiation with an energy source (light, magnetic field, radiofrequency application), the surrounding tissue is heated to 39–45 °C, which induces cell stress that triggers the unfolded protein response (UPR) [79]. The UPR can lead to immunogenic cell death (ICD); the release of tumor-associated antigens (TAAs) and danger-associated molecular patterns (DAMPs); and activation of myeloid cells, especially dendritic cells (DCs), that prime T cells to initiate an antigen-specific adaptive anti-cancer immune response executed mainly by CD8+ cytotoxic T cells [70]. (B) RNA-binding nanoparticles are loaded with siRNA targeting lactate dehydrogenase A (LDH). The functional knockdown of LDH minimizes lactate production, reversing the acidic pH of the TME, with a subsequently enhanced immune response and a reduced tumor neoangiogenesis [75]. (C) Nanoparticles for TAM-specific delivery of TLR agonists [80,81] or small molecules inhibiting colony-stimulating factor 1 receptor (CSF-1R) [82] can shift the immune-suppressive M2 phenotype into an inflammatory M1 phenotype promoting CD8+ T cell mediated killing of cancer cells. (D) Artificial antigen presenting cells (aAPCs) express at least one peptide-MHC complex (for example, loaded with a highly expressed intracellular TAA) and a costimulatory signal, for example, anti-CD28 for effective T cell priming. They can also be further modified and loaded with cytokines or ICIs. In comparison with their cellular counterparts, they have the benefit of maintaining an “always on” state that cannot be inactivated [83], allowing for an effective anti-cancer CD8+ T cell priming in the otherwise immunosuppressive TME. Abbreviations: dendritic cell (DC), myeloid-derived suppressor cell (MDSC), cancer-associated fibroblast (CAF), regulatory T cell (Treg), siRNA directed to LDH (siLdh).