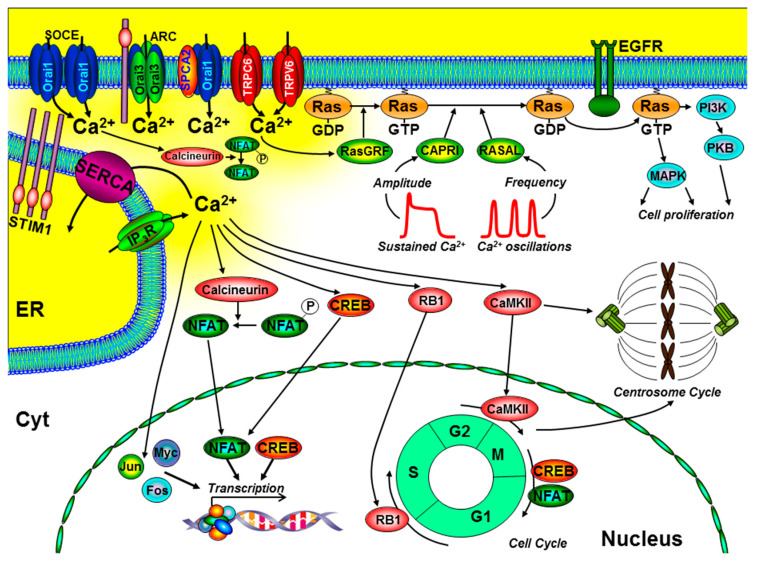
Figure 2.
The role of key components of the Ca2+ signalling machinery in the control of cell proliferation and the cell cycle. Ca2+ entry through store-operated Ca2+ channels (SOCE; consisting of endoplasmic reticulum (ER) stromal interacting molecule (STIM)1 and Orai1), arachidonate-regulated Ca2+ channels (ARC; consisting of plasma membrane STIM1 and Orai1/Orai3 heteropentamers), transient receptor potential channel (TRPC)6, TRPV6, and the non-SOCE (plasma membrane secretory pathway ATPase (SPCA)-regulated Orai1) have all been implicated to regulate cell proliferation. Specific spatiotemporal patterns of Ca2+ signals can differentially regulate gene transcription (via calcineurin/nuclear factor of activated T cells (NFAT), CAMKII/cAMP response element-binding protein (CREB), immediate early genes (Jun, Myc, and Fos)), and the Ras/ERK signalling pathway. Localised Ca2+ entry can specifically activate the Ras guanine exchange factor (Ras-GEF), Ras-GRF, which converts the inactive Ras-guanosine diphoshpate (Ras-GDP) to the activate Ras-guanosine triphoshpate (Ras-GTP). Moreover, the amplitude of Ca2+ signals can specifically activate CAPRI and the frequency of Ca2+ oscillations can specifically activate RASAL, both of which are Ras guanine activating proteins (Ras-GAPs) that inactivate Ras-GTP. Ca2+ also has an important role in the control of the cell cycle and centrosome cycle. Specifically, Ca2+-dependent activation of retinoblastoma-1 (RB1) regulates G1/S phase transition, CaMKII regulates G2/M phase transition, and CREB and NFAT can regulate M/G1 transition. Additional abbreviations include EGFR, epidermal growth factor receptor; PI3K, phosphinositide-3 kinase; PKB, protein kinase-B; MAPK, mitogen-activated kinase; cell cycle phases: G1 and G2, gap; S, synthesis; M, mitosis.