Figure 3.
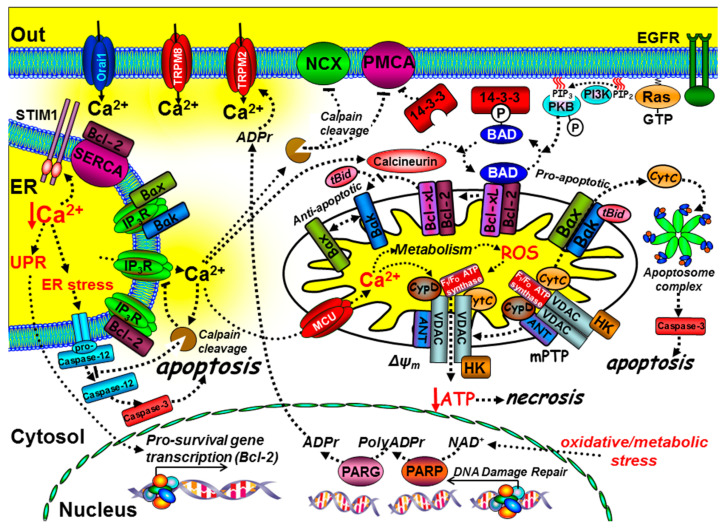
The role of key components of the Ca2+ signalling machinery in cell death and immortality. Ca2+ mediates intrinsic cell death at both the mitochondria and ER. At the mitochondria, tBid binds to and promotes Bax and Bak oligomerisation into pores that release cytochrome C, which binds to the apoptosome complex and activates the executioner caspases, such as caspase-3, leading to the “point-of-no-return” apoptotic cascade. The anti-apoptotic proteins, Bcl-2 and Bcl-xL, can prevent the t-Bid/Bax/Bak interaction, thereby preventing apoptosis. The pro-apoptotic protein Bad binds to Bcl-2/Bcl-xL, thereby preventing their interaction with the t-Bid/Bax/Bak complex, thus promoting apoptosis. Phosphorylation of Bad, via growth factor receptor-mediated activation of protein kinase-B (PKB), causes Bad to dissociate from the mitochondria and bind to 14-3-3 protein. Sustained Ca2+ overload can activate calcineurin, which dephosphorylates Bad, allowing it to sequester the anti-apoptotic proteins, Bcl-2/Bcl-xL. Ca2+ uptake into the mitochondria, via the mitochondrial Ca2+ uniporter (MCU), can lead to the production of reactive oxygen species (ROS). Ca2+ and ROS can activate the permeability transition pore (mPTP), loss of the mitochondrial membrane potential (ΔΨm), ATP depletion and necrosis. Ca2+ overload can also activate calpain and cleavage of the PMCA, Na+/Ca2+-exchange (NCX), and inositol 1,4,5-trisphosphate receptors (IP3Rs). Bax, Bak, and Bcl-xL can also bind to and inhibit IP3Rs. Bcl-2 can bind to and inhibit SERCA, reducing ER Ca2+.