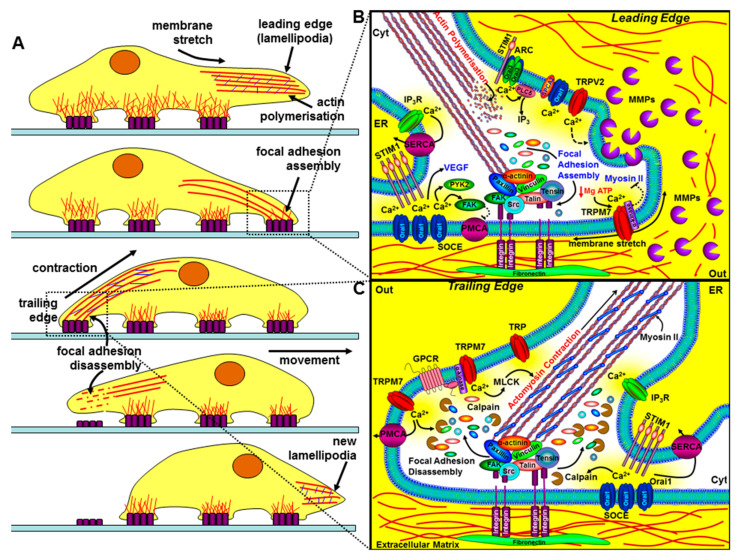
Figure 4.
The role of key components of the Ca2+ signalling machinery in cell migration and invasion. The left panel (A) depicts the sequential steps of a migrating cell. The bottom part of the cell makes contact with the extracellular matrix (ECM) via integrins (purple rectangles), which couple the ECM to the actin cytoskeleton. The leading edge involves specialised membrane protrusions (lamellipodia/invadapodia) containing matrix metalloproteinases (MMPs), actin polymerisation, the assembly of focal adhesions, and the formation of new integrin-mediated contact sites. The trailing edge requires focal adhesion disassembly, actomyosin contraction, and the loss of rear-end contact sites. The right panels (B and C) show a magnified view of how key components of the Ca2+ signalling machinery regulate the leading edge (B) and the trailing edge of a migrating cell (C). At the leading edge, specific localised Ca2+ entry, amplified by Ca2+ release through IP3Rs, can lead to focal adhesion assembly via the Ca2+-dependent activation of calmodulin-dependent kinase (CaMKII), proline-rich tyrosine kinase (PYK2), and focal adhesion kinase (FAK). Ca2+ entry through store-operated Ca2+ channels (SOCE; consisting of ER STIM1 and Orai1), TRPM7, TRPV2, arachidonate-regulated Ca2+ channels (ARC; consisting of plasma membrane STIM1 and Orai1/Orai3 heteropentamers), and the non-SOCE (plasma membrane SPCA-regulated Orai1) have all been implicated in the regulation of cell migration and invasion. Membrane stretch can activate Ca2+ entry through TRPM7 and the α-kinase domain of TRPM7 can also phosphorylate myosin-IIA heavy chain and inhibit actomyosin contraction and lead to cell spreading. Ca2+ entry through TRPV2 has also been shown to regulate the release of MMPs and the focal degradation of the ECM. At the trailing edge, Ca2+ entry through SOCE and TRPM7 channels leads to the coordinated Ca2+-dependent activation of m-calpain, leading to focal adhesion disassembly, and myosin light chain kinase activation, leading to actomyosin contraction.