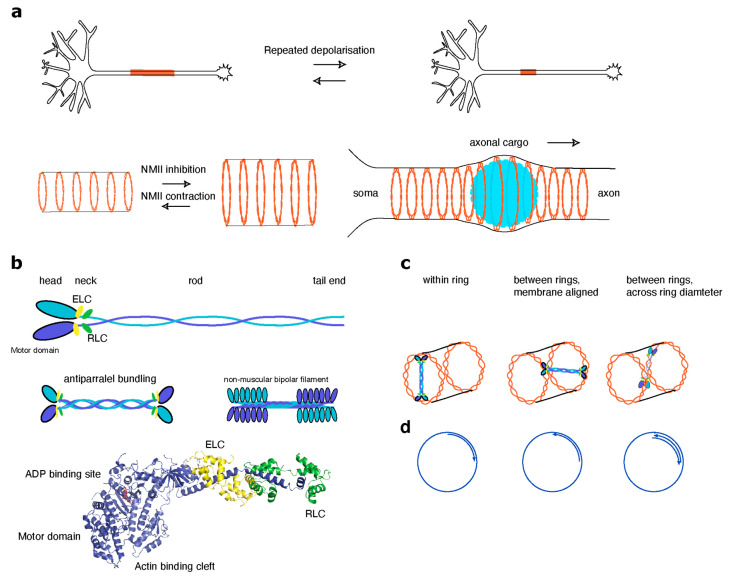
Figure 2.
Organization and function of non-muscle myosin II (NMII). (a, top) Repeated depolarization leads to proximal shortening of the AIS and subsequent extension towards the distal axon. NMII activity is necessary for this process as blebbistatin completely blocks the activity. (a, bottom) NMII activity controls the axonal diameter. Inhibition of NMII by blebbistatin leads to an increase in axon diameter, indicating that NMII holds the membrane-associated cytoskeleton (MSK) under constant tension. In addition, NMII has been shown to be implicated in cargo trafficking along the AIS. The size of large cargo (e.g., autophagosomes, mitochondria, endosomes or lysosomes) can exceed the diameter of the axon. Passage of this large axonal cargo causes a transient radial expansion of the axon followed by constriction, which depend on myosin II activity. (b, top) Schematic organization of NMII. NMII exists as a hexamer that consists of two copies each of elongated heavy chains, two regulatory light chains (RLC) and two essential light chains (ELC) that stabilize the heavy chain structure. The heavy chain is composed of an N-terminal motor domain, a neck domain, which interacts with both light chains, an α-helical rod domain and a C-terminal tail. (b, middle) The hexameric units further bundle both in a parallel and antiparallel manner into bipolar structures that can pull actin filaments together. (b, bottom) Crystal structure of the motor and neck domains of NMII interacting with ELC and RLC. The motor domain contains the actin binding cleft where NMII interacts with actin. Shown in red is ADP bound at the nucleotide binding site. Cycling from ATP to ADP at the nucleotide binding site leads to conformational changes in the actin binding cleft, which modulate the interaction of NMII with actin. (c) Models of a possible spatial relationships between NMII and actin rings. The length of an active two-headed myosin motor complex is around 300 nm, while the distance between actin rings is only 190 nm. (c, left) NMII crosses the diameter of a single actin ring. Alternatively, NMII could cross link two neighboring rings with an angle deviant from 90°, which can be achieved when myosin motors connect neighboring actin rings in a one-dimensional lattice (as spectrin) (c, middle) or across the axonal volume (c, right). (d) Polarity of actin filaments in ring-forming actin braids. Force generated by NMII induces filament sliding within the braid that results in constriction or expansion of the ring. However, as myosin II steps towards the barbed end of an actin filament, the orientation of filaments within a single braid and filament polarity with respect to the neighboring rings are important. The NMII mechanism can work when filaments within a single ring are parallel (d, left) but the adjacent braids have opposite polarity (d, middle) or when two filaments within the same braid have an opposite polarity (c, right).