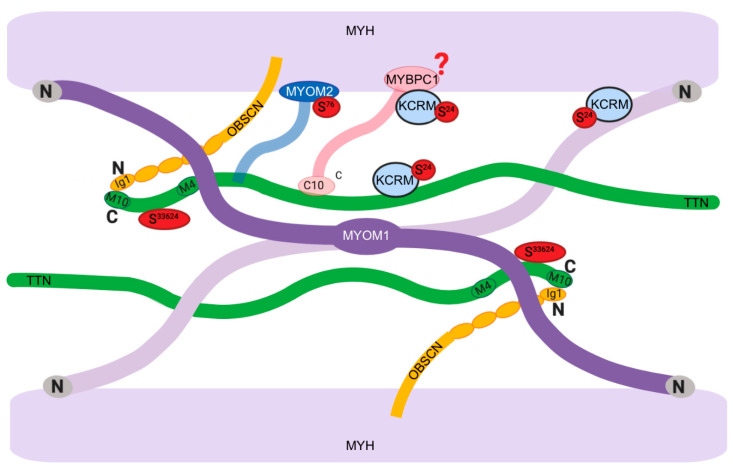
Figure 7.
Diurnal differences in M-band phosphorylation. Diagrammatic representation of M-band protein interactions redrawn from [38]. Myomesin 1 (MYOM1) and obscurin (OBSCN) are principal M-band proteins [39]. The N-terminal regions of MYOM1 interact with myosin heavy chain (MYH), titin (TTN), and muscle creatine kinase (KCRM), whereas the C-terminal regions of MYOM1 dimerize in an anti-parallel arrangement that corresponds with the M-bridges [40]. MYOM1 interacts with the C-terminal regions of TTN within the M-band. The TTN kinase domain (resides 32,178–32,432) includes the regulatory Y32341 residue [41], whereas we discovered that the phosphorylation of S33624 of TTN is greater in the morning than evening. Myomesin 2 (MYOM2, formally known as M-Protein) interacts with the light meromyosin (LMM) region of MYH and the C-terminal region of TTN [42]. The phosphorylation of MYOM2 S76 disrupts the binding of MYOM2, and LMM [43] and is more prevalent after exercise in the morning compared to evening. Myosin binding protein C (MYBPC1) localizes to the M-band through its interaction with obscurin [44]. MYBPC1 is required for the binding of muscle creatine kinase (KCRM) to the M-band region. MYBC1 exhibits differences in post-translational state between morning and evening, which are likely to be phosphorylation [45]. KCRM S24 phosphorylation is greater in the morning than evening. The N-terminal region of KCRM is necessary for the creatine/phosphocreatine- and pH-dependent interaction of KCRM with the M-band [46].