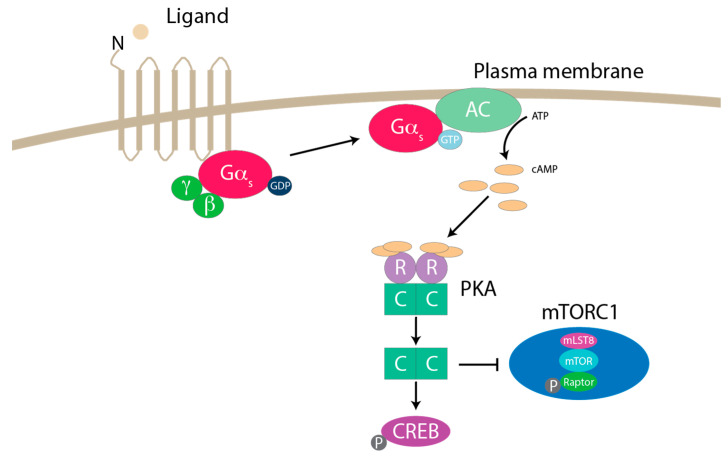
Figure 4.
GPCR inhibition of mTORC1. Activation of Gαs-coupled GPCRs inhibit mTORC1 through the activation of PKA. The GPCR effector is a heterotrimeric G-protein made of three subunits (Gα, Gβ and Gγ). G-proteins are inactive in the GDP-bound state. GPCRs function as a receptor-catalyzed GEF to activate Gα subunit and separate them from the Gβγ dimer through conformational change. GTP hydrolyzing to GDP is the rate limiting step for Gα activity. Gα GDP-bound subunit then rejoins the βγ dimer until the next activating cycle. The GTP-Gαs subunit can interact and turn on AC. AC is able to convert ATP cAMP. G-protein signaling induces cAMP, activating second messenger kinases such as PKA. PKA is a holoenzyme made of two regulatory subunits (R) and two catalytic subunits (C). R subunits have cAMP binding motifs. Two molecules of cAMP bind each R subunit to release and activate the C subunit of PKA. A well-known phosphorylation target of PKA is CREB at Ser 133. PKA can phosphorylate Raptor on Ser 791 to inhibit mTORC1 activity. adenylate cyclase (AC); 3′,5′-cyclic adenosine monophosphate (cAMP); G-protein coupled receptors (GPCRs); protein kinase A (PKA).