Figure 3.
Priming Promotes Cell Death in Response to Cytosolic LPS Independently of CASP11 Expression
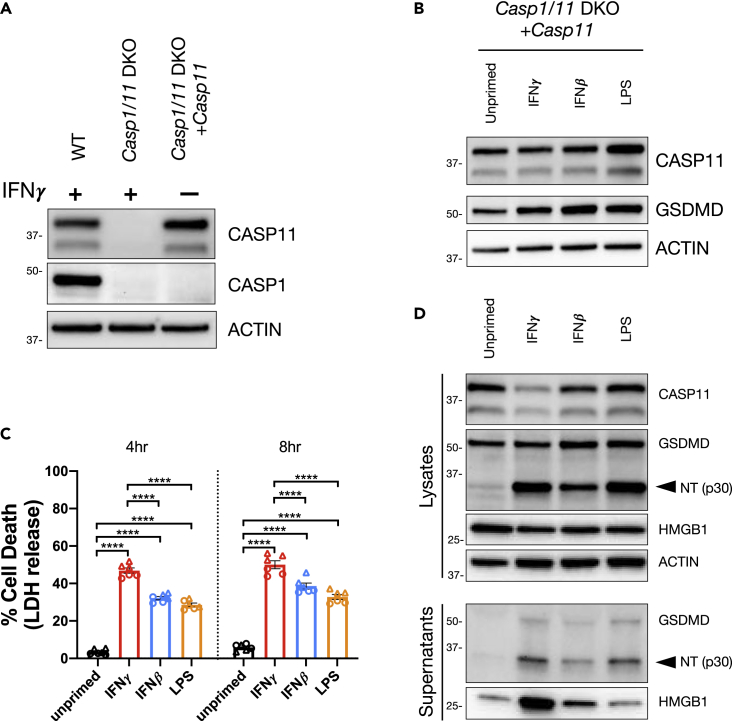
A constitutive CASP11-expressing cell line was generated by transducing a CASP11 expression vector into Casp1,Casp11-CRISPR/Cas9 DKO RAW cells (Casp1/11 DKO).
(A) Cell lysates were collected from WT, Casp1/11 DKO, and the constitutive cell line (Casp1/11 DKO + Casp11) and analyzed by western blot to determine CASP1 and CASP11 expression. WT and Casp1/11 DKO were treated with IFNγ (100 U/mL) for 16 h.
(B) The constitutive CASP11-expressing cell line was primed for 16 h overnight with the following treatments; unprimed (N/A), IFNγ (100 U/mL), IFNβ (1,000 U/mL), or LPS (10 ng/mL). Lysates were collected to determine the effects of priming on CASP11 expression by western blot.
(C and D) The constitutive CASP11-expressing cell line was primed as described above and CASP11 inflammasome activation was triggered by LPS (E. coli 0111:B4, 50 μg/mL) transfection with FuGENE HD. (C) At 4 and 8 h following inflammasome activation, supernatants were collected to measure release of LDH for percent cell death calculations. (D) Alternatively, supernatants and lysates were collected 3 h post transfection to monitor for the cleavage and release of inflammasome-related proteins by SDS-PAGE and western blot. Molecular weight marker positions are shown to the left of each blot, and arrows indicate a cleavage product. Bar graphs show the mean value +/− SEM along with individual data points pooled from independent experiments depicted with different shapes (C). Data were pooled from two (C) independent experiments or are representative of three (A and B) or two (D) independent experiments.
Statistical analysis performed using a two-way ANOVA and Tukey's multiple comparisons test; ∗∗∗∗ <0.0001. See also Figure S3.