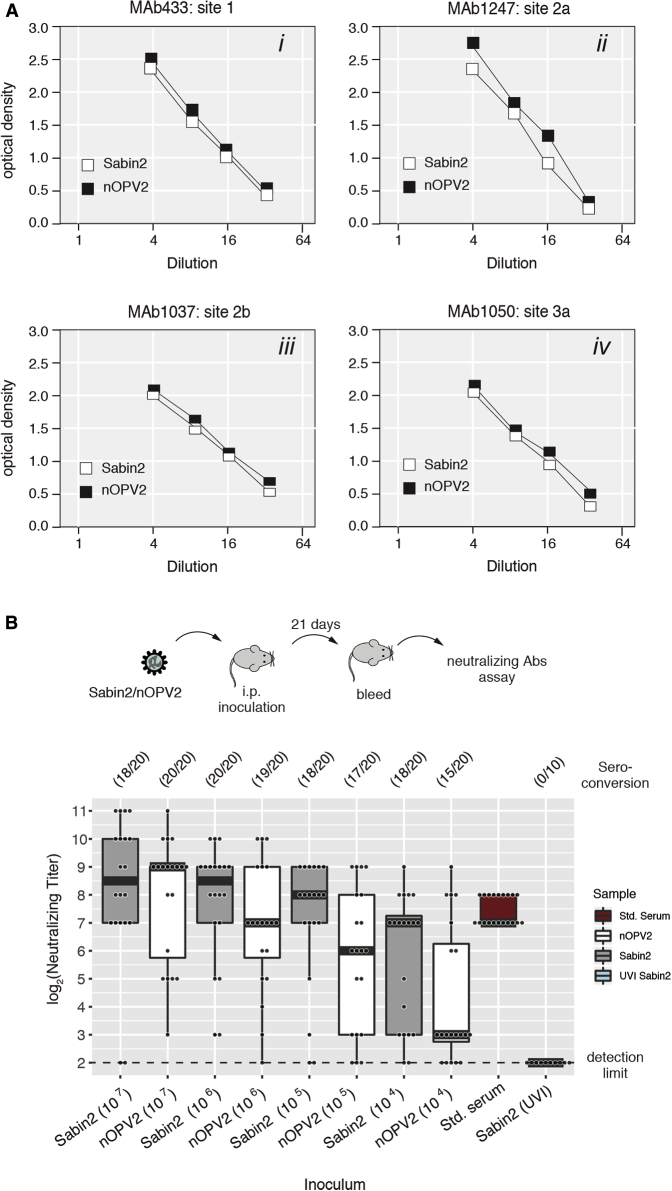
Figure 6.
Antigenicity and Immunogenicity of nOPV2 in Mice
(A) Reactivities of nOPV2 with monoclonal antibodies against four antigenic sites on the poliovirus type 2 virion were compared to Sabin2 reactivity in ELISA assays.
(B) Interferon-receptor knockout, transgenic mice expressing the human poliovirus receptor (IFNAR−/− TgPVR21) were inoculated intraperitoneally with a range of doses (104–107 PFU) of Sabin2 and nOPV2. Mice injected with 107 pfu of ultraviolet light inactivated (UVI) Sabin2 were included as controls. Ten mice were used per condition for Sabin2 and nOPV2 and five mice for Sabin2 (UVI) in each experiment. Data shown were collected from two experiments. Titers of neutralizing antibody (NT) in sera at day 21 were determined by NT assay as described in STAR Methods. Box-and-whisker diagram represents the neutralizing antibody response for each condition. Bars in boxes represent median antibody titers. Whiskers represent the range of non-outliers observations (less than 2.5∗ IQR from the median). Overlapping dots represent neutralizing antibodies values obtained for each individual mouse. Statistical analysis (two-tailed Mann-Whitney U test) was performed to compare difference in titers of neutralizing antibody induced by Sabin2 and nOPV2: (p value: 0.53707, 0.1564, 0.0775, and 0.0731 for 107, 106, 105, and 104, respectively). Std is serum from a human subject vaccinated with OPV. At the top of the graph, seroconversion frequency (number of individuals that seroconverted over total).