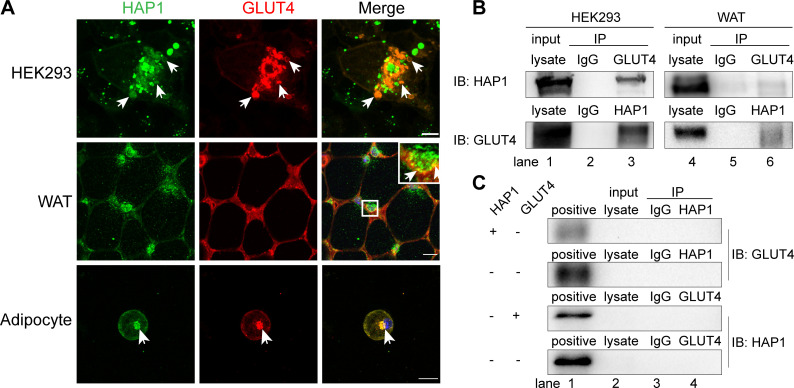
Figure 4.
Huntingtin-associatedprotein 1 (HAP1) interacted with glucose transporterisoform 4 (GLUT4) in vivo and in vitro. (A) HAP1 and GLUT4 were colocalized in an intracellular location. Top row: HAP1-CFP and GLUT4-mCherry cotransfected HEK293 cells. Scale bar, 10 µm. Middle row: Double staining of mouse adipose tissue with HAP1 (green) and GLUT4 (red). Scale bar, 100 µm. Bottom row: Double staining of wild-type (WT) primary adipocytes with HAP1 (green) and GLUT4 (red). Scale bar, 20 µm. The nuclei (blue) were stained with 4′,6-diamidino-2-phenylindole (DAPI). (B) Co-immunoprecipitation (Co-IP) assay was applied for identification of the interaction between HAP1 and GLUT4. Detection of HAP1 and GLUT4 in HEK293 cells cotransfected with HAP1 and GLUT4 plasmids (left) and mouse adipose tissue (right) by western blotting. Lanes 1 and 4 for the positive control of total protein lysate, lanes 2 and 5 for the IgG as negative control, and lanes 3 and 6 for the Co-IP sample. (C) Co-IP was applied for the identification of endogenous HAP1 and GLUT4 expression of HEK293 cells. HEK293 cells were with or without HAP1 or GLUT4 plasmid, and the other protein was detected by western blotting. Lane 1 for the positive control of HEK293 cells cotransfected with HAP1 and GLUT4 plasmids’ total protein lysate, lane 2 for total protein lysate of sample for Co-IP, lane 3 for the IgG as negative control and lane 4 for the Co-IP sample. IB, immunoblotting; IP, immunoprecipitation; WAT, white adipose tissue.