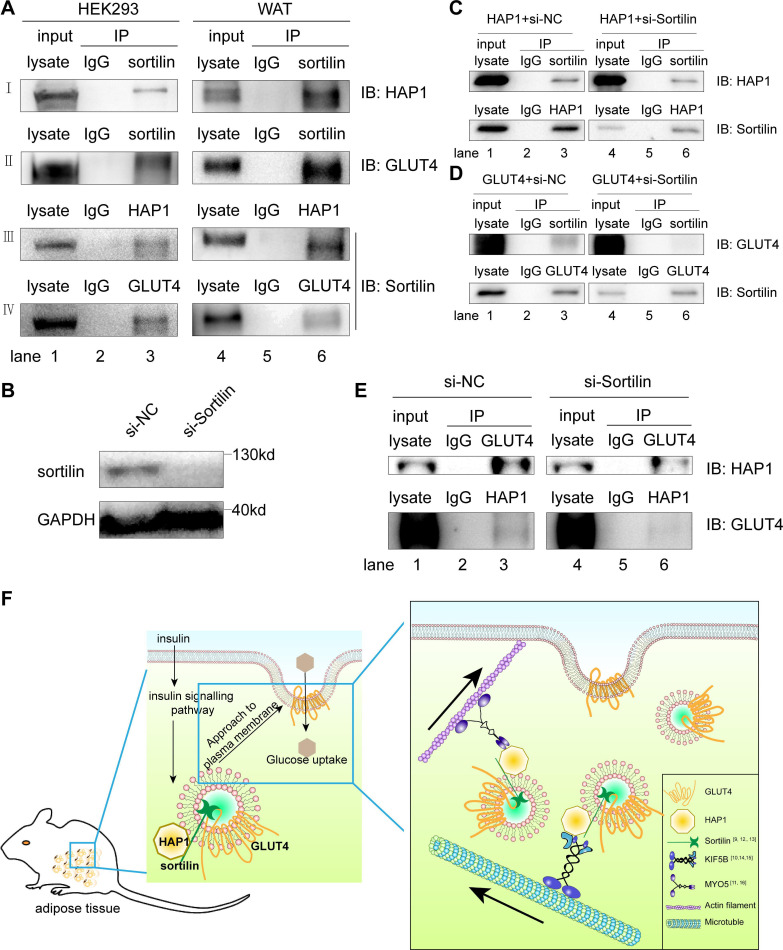
Figure 5.
Huntingtin-associatedprotein 1 (HAP1) formed a protein complex with glucose transporterisoform 4 (GLUT4) via sortilin. (A) Co-immunoprecipitation (Co-IP) was applied for identification of the interaction among HAP1, GLUT4 and sortilin. Detection in HEK293 cells cotransfected with HAP1 and GLUT4 plasmids (left) and mouse adipose tissue (right) by western blotting. (B) Sortilin protein expression was detected after 48-hour interference. (C) HEK293 cells were transfected with HAP1 plasmids and interfered with or without si-Sortilin, and proteins were collected for Co-IP. (D) HEK293 cells were transfected with GLUT4 plasmids and interfered with or without si-Sortilin, and proteins were collected for Co-IP. (E) HEK293 cells were cotransfected with HAP1 and GLUT4 plasmids; meanwhile, sortilin was reduced by small interfering RNA (siRNA). Interaction between HAP1 and GLUT4 was detected in sortilin-siRNA or sortilin-scramble-treated HEK293 cells. Lanes 1 and 4 for the positive control of total protein lysate, lanes 2 and 5 for the IgG as negative control, and lanes 3 and 6 for the Co-IP sample. (F) The schematic model and proposed mechanism of HAP1, GLUT4 and sortilin–protein complex involved in the insulin-stimulated GLUT4 translocation. In mouse adipocytes, HAP1 forms a complex with GLUT4 via sortilin and interacts with motor proteins, promoting the GLUT4 transport along cytoskeleton. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IB, immunoblotting; IP, immunoprecipitation; KIF5B, kinesin family motorprotein 5; MYO5, myosin 5; NC, negative control; WAT, white adipose tissue.