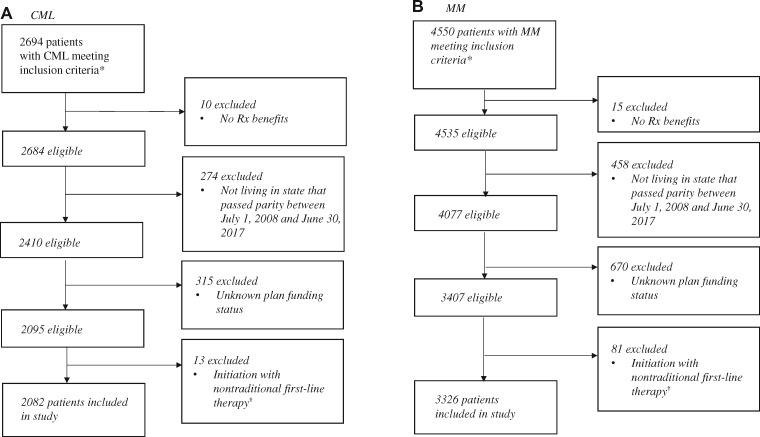
Figure 1.
Sample flow diagram. A) Patient selection for individuals with chronic myeloid leukemia and (B) Patient selection for individuals with multiple myeloma. Source: Authors’ analysis of Health Care Cost Institute Claims, 2008–2017. *Inclusion criteria: filled an orally administered anticancer drug of interest; was continuously enrolled in a health plan for 3 months pre- and 6 months postinitiation; aged 64 years or younger at index fill. We used an intent-to-treat approach, ignoring switching plan funding status changes postindex treatment (<1% of individuals meeting inclusion criteria switched plan funding types during the study period). †We excluded individuals with chronic myeloid leukemia (CML) who initiated bosutinib (n = 9) or ponatinib (n = 4) and individuals with multiple myeloma (MM) who initiated pomalidomide (n = 81) because these therapies are not typically used in the first-line setting for the disease of interest.