Figure 3.
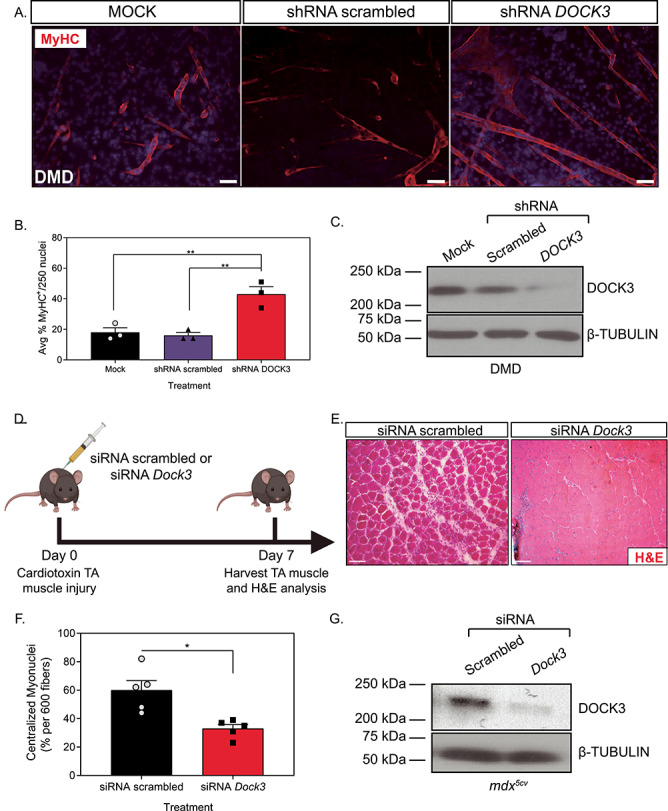
Knockdown of DOCK3 in DMD patient muscle cells and mdx5cv mice increases myogenic differentiation and improves pathology. MyHC staining reveals that the knockdown of DOCK3 protein in human DMD primary myotubes increases myogenic fusion indices. (A) Immunofluorescence of MyHC (MF-20 antibody, DSHB Iowa, red) and nucleus marker (DAPI, blue) of Day 7 differentiated human DMD primary myotubes either transduced with mock (control), shRNA scrambled (negative control) and shRNA DOCK3 lentiviral particles [transduced at a multiplicity of infection (MOI) of 10]. Scale bar = 50 μm. (B) Summary graph of average myogenic fusion indices as calculated by percentage of nuclei inside of MyHC+ cells out of 250 nuclei, as previously described (68). DMD primary myotubes transduced with shRNA DOCK3 demonstrated significantly higher amount of myogenic fusion. (n = 3 experimental replicates, one-way ANOVA with Tukey’s correction, *P < 0.05). (C) Western blot analysis of whole cell lysates taken from Day 7 differentiated DMD myotubes transduced with mock (control), shRNA scrambled (shRNA internal control) or shRNA DOCK3 knockdown lentiviral particles, demonstrating successful knockdown of DOCK3 protein expression. (D) Schematic showing co-injection in mdx5cv mice of cardiotoxin (10 μM ctx) as well as either siRNA double-stranded (ds) oligos to inhibit mouse Dock3 mRNA (siRNA Dock3) or a scrambled ds oligo (siRNA scrambled; control). Experiment was performed in each leg and mice were evaluated 7 days post ctx injury. (E) Representative H&E staining of the siRNA-injected mdx5cv muscles 7 days post ctx. Scale bar = 50 μm. (F) Summary graph showing reduced centralized myonuclei when mdx5cv mice were injected with siRNA Dock3 (n = 5, student’s t-test, two-tailed, *P < 0.05). (G) Western blot analysis of whole muscle lysates from siRNA scrambled and siRNA Dock3 muscle demonstrating successful knockdown of Dock3 protein.
