Figure 2.
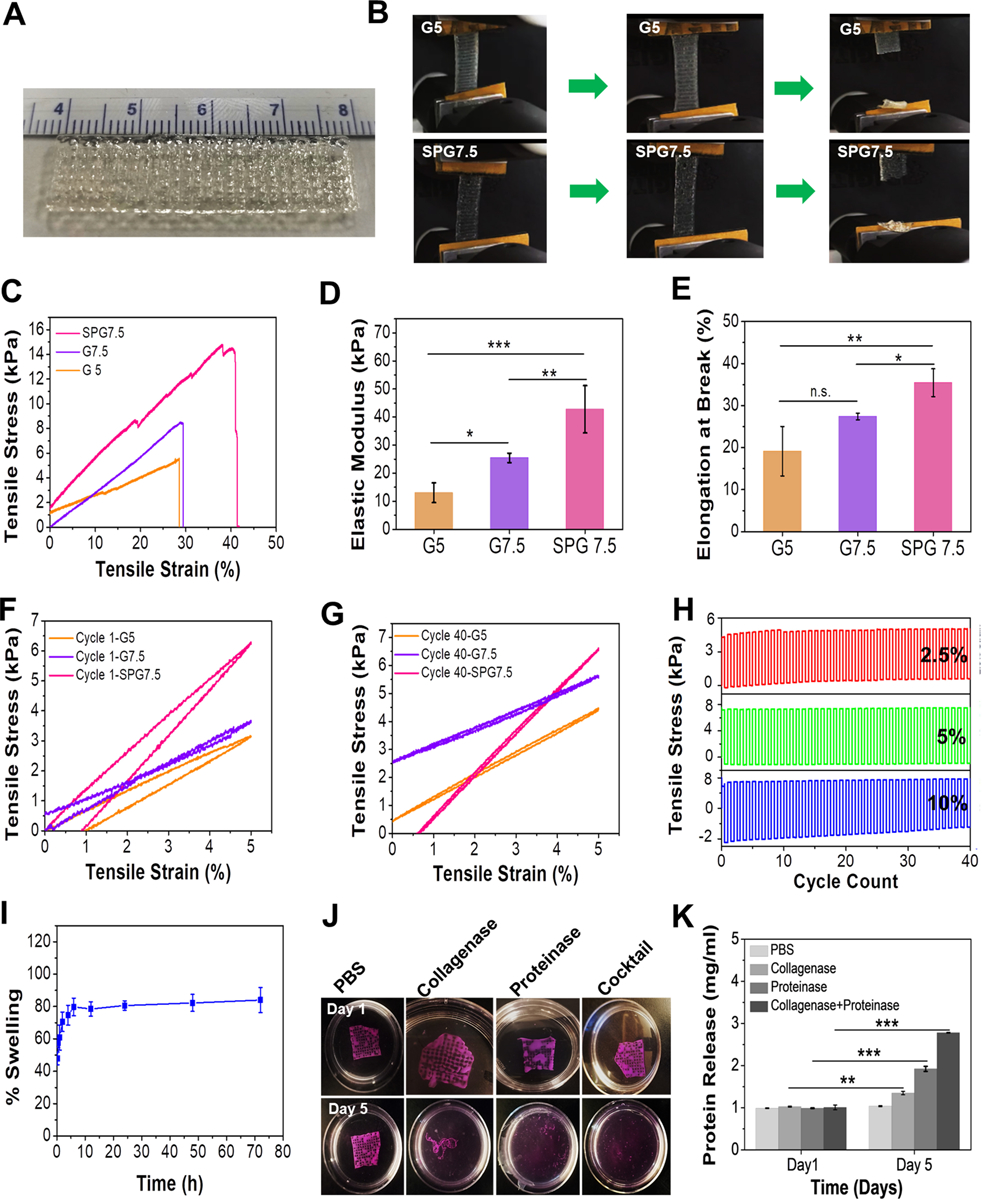
Characterization of 3D printed constructs using the optimized ink composition. (A) Large scale and easy-to-handle anisotropic 3D printed construct fabricated using SPG7.5 ink. (B) Images of the 3D printed construct with 5% GelMA inks as control (G5) and SPG7.5 ink under tensile tests. (C) Stress-strain curves, (D) evaluation of elastic moduli, and (E) percentage elongation at break of the bioprinted constructs fabricated using G5, G7.5 and SPG7.5 inks (n=4,*** p ≤ 0.001, **p ≤ 0.01 and *p ≤ 0.05). (F-G) Cyclic tensile tests of the 3D printed constructs using G5, G7.5 and SPG7.5 inks at 5% tensile strain for cycle 1 and cycle 40 (n=4). (H) Cyclic tensile tests of the 3D printed construct using SPG7.5 ink at 2.5% (red), 5% (green) and 10% (blue) strain for 40 cycles (n=4). (I) Swelling behavior of the 3D printed construct using SPG7.5 ink in DPBS (n=4). (J) Enzymatic degradation behavior of the 3D printed constructs in collagenase type II, proteinase K, and a cocktail (mixture of collagenase type II and proteinase K). Constructs kept in DPBS alone were used as controls. (K) Bradford assay for determing the amount of protein released as a result of degradation of the printed constructs in the presence of enzymes (n=3; *** p ≤ 0.001 and **p ≤ 0.01).
