Figure 5.
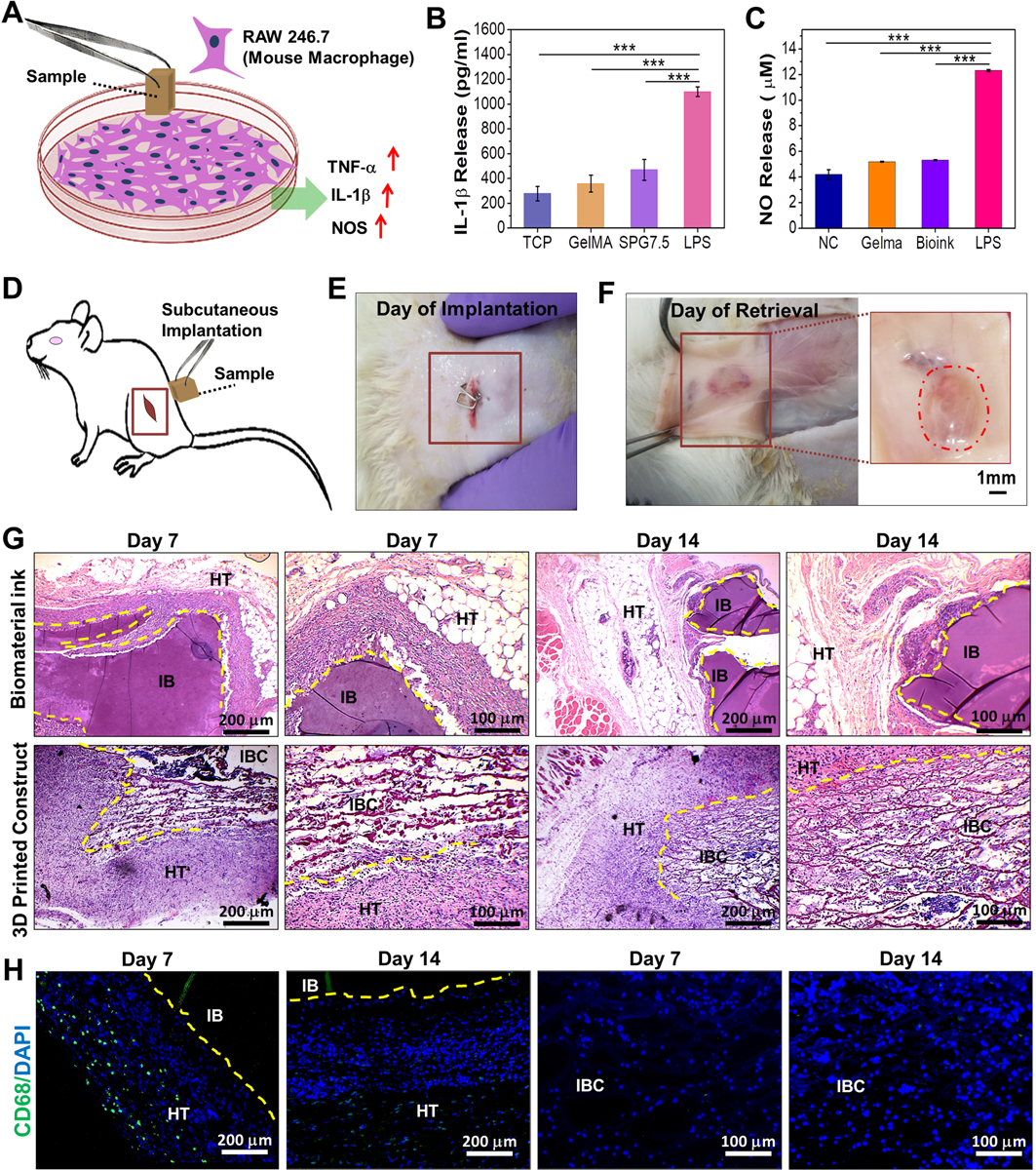
Immunocompatibility assessment of the biomaterial ink and 3D printed constructs. (A) Schematic depicting the release of pro-inflammatory molecules by mouse macrophage (RAW 246.7) when exposed to the 3D printed samples. Quantification of (B) IL-1β and (C) NO (nitric oxide) release by macrophages as determinants for in vitro immunocompatibility of the 3D printed SPG scaffolds compared to control samples (n=3; *** p ≤ 0.001) (D) Schematic depicting the implantation of samples in the left subcutaneous pocket of Sprague-Dawley rats. Images depicting the 3D printed scaffolds (E) after implantation and (F) on day 14 of retrieval (n=4). In vivo immunocompatibility assessment via (G) H&E staining of the implanted biomaterial ink and 3D printed scaffolds post retrieval at both low and high magnifications. Host cell infiltration was observed for IBC on subcutaneous implantation on day 7 and day 14. (H) CD68 immunostaining for determining the infiltration of macrophages post implantation of the bioprinted scaffolds on days 7 and 14 where HT stands for host tissue; IB stands for implanted biomaterial ink and IBC stands for implanted biomaterial ink based 3D printed construct.
